Abstract
The transient receptor potential (TRP) proteins are six transmembrane-containing subunits that combine to form cation-selective ion channels. TRP channels are present in yeast, Drosophila, Caenorhabditis elegans, and mammals. They are widely distributed and sense local changes in stimuli ranging from light to temperature and osmolarity. Mammals contain at least 22 distinct genes encoding these ion channels. This summary article presents an overview of the molecular relationships among the TRP channels and a standard nomenclature for them, which is derived from the IUPHAR Compendium of Voltage-Gated Ion Channels.1 The complete Compendium, including data tables for each member of the TRP channel family, can be found at http://www.iuphar-db.org/iuphar-ic/.
Introduction
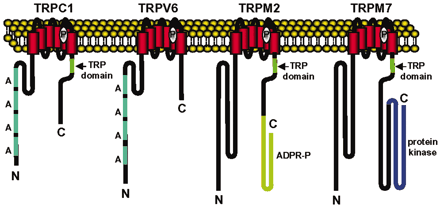
The mammalian TRP2 ion channels are encoded by at least 22 channel subunit genes, rising to >30 if polycystic kidney (PKD, TRPP) and mucolipins (TRPML) are included. TRP channel primary structures predict six transmembrane (6TM)-spanning domains with a pore domain between the fifth (S5) and sixth (S6) segments and both C and N termini located intracellularly. This architecture is a common theme for hundreds of ion channels present in life forms ranging from bacteria to mammals. The mammalian TRP channel family is united primarily by structural homology within the transmembrane-spanning domains (Fig. 1), but overall sequence identities between members can be as low as 20%. Other features include a 25-amino acid motif (TRP domain) containing TRP box (EWKFAR) just C-terminal to the sixth transmembrane segment. The TRP domain and box are present in all TRPC channel genes but not in all TRP channel genes. The N-terminal cytoplasmic domain of TRPC, TRPV, and ANKTM (TRPA) channels contain ankyrin repeats, whereas the TRPC and TRPM contain proline-rich regions in the region just C-terminal to the predicted 6TM segment (Fig. 2). At present, no one feature other than overall 6TM architecture and homology define the TRP family. Thus, we expect that the definition of TRP channels will evolve as functions and structures are clarified.
Phylogenetic relationship in the TRP protein family. The evolutionary tree is calculated by the neighbor-joining method (Saitou and Nei, 1987). The TRPP and TRPML subfamilies are not detailed in this review.
Architecture of TRP channels is that of the broader class of six transmembrane-spanning ion channels. S1–S6 are transmembrane domains. A represents ankyrin repeats.
Transient receptor potential (trp) ion channel subunit genes were first defined in the Drosophila visual system. In the trp mutation, the light response (receptor potential) decays during prolonged exposure to bright light. TRP-deficient flies are blinded by intense light because sustained Ca2+ entry via TRP ion channels and subsequent Ca2+-dependent adaptation is disrupted. Three genes (TRP, TRPL, TRPγ) in Drosophila encode TRP channels mediating fly vision and other unknown functions. Genetic approaches in flies have not resolved the mechanism of TRP activation, but they confirm the importance of phospholipase Cβ (PLCβ) and other components of the phosphatidylinositol pathway (Harteneck et al., 2000; Clapham et al., 2001; Montell et al., 2002; Hardie, 2003).
Structural Features
The 2TM structure of a bacterial K+ channel (KcsA) is analogous to the S5 and S6 domains joined by a short pore α helix of the 6TM architecture (Doyle et al., 1998). KcsA channel is a tetramer of 2TM-spanning α helices. The helices corresponding to S5 face the lipid membrane whereas the helices corresponding to S6 line the pore. At both inner and outer membrane faces, layers of aromatic amino acids form a cuff around the pore. In KcsA the selectivity filter is a narrow region near the outer face of the membrane lined by the carbonyl backbone of five conserved amino acids. These amino acids are not present as a group in the largely nonselective TRP channels. In KcsA, rings of carbonyl oxygens act as surrogate waters to coordinate the dehydrated K+ ions in the channel. The rest of the S5- and S6-spanning regions are likely to be analogous to KcsA in which the narrow channel in the selectivity filter rapidly broadens in hourglass fashion. The four short pore α helices focus their helix dipole negative electrostatic fields on the cavity to shield the cation from the hydrophobic lipid environment. The S6 base lines the rest of the channel on its way to the cytoplasm. The S6 segment and the C-terminal amino acids extending into the cytoplasm are where the interesting gating features of TRP channels are likely to emerge. The most conserved regions between the three TRP subfamilies are in the S6 domain.
The detailed structure of the S1–S4 segments of the 6TM channels is not available, but mutagenesis data provide some clues about their functions. The S4 segment in voltage-sensitive channels contains at least four charged arginines or lysines that convey voltage changes across the membrane into movement of the helix, somehow gating the pore by moving this S4 helix. The TRP channels are very weakly voltage-dependent and lack the full complement of charged amino acids in the S4 domain.
TRP Channel Functional Features
All TRP channels are nonselective with PCa/PNa ≤ 10, with the exception of the monovalent-selective TRPM4,M5, and the Ca2+-selective (PCa/PNa > 100) TRPV5,V6.
TRP channels do not have the sharp voltage sensitivity of the 24 membrane-spanning CaV or NaV families (Fig. 3). Thus, upon opening, they depolarize cells from their resting membrane potentials (roughly -70 mV in most mammalian cells) to around 0 mV. In short, they depolarize cells and raise intracellular Ca2+ and/or Na+.
Current-voltage relationships of various TRP channels; a, TRPC1 + TRPC5 heteromer; b, TRPM5; c, TRPV5, TRPV6.
Two common signal transduction pathways regulating the release of intracellular Ca2+ are the G protein-coupled and the tyrosine kinase activation of PLC. PLC hydrolyzes phosphatidylinositol 4,5-bisphospate (PIP2) to form inositol 1,4,5-trisphosphate (IP3) that opens the IP3 receptor (IP3R), and liberates Ca2+ from the endoplasmic reticulum (Clapham, 1995). Accompanying these chains of events, and not necessarily linked to Ca2+ store (ER) depletion, is activation of the TRP channels. The details of these mechanisms are incompletely understood at present. The strongest associations between the phosphatidylinositol pathway and TRPs involve PLCβ and PIP2. Based on Drosophila TRPs, elements of these signal transduction pathways are linked by scaffolding proteins (Montell, 1999).
Putney (1977) proposed that emptied Ca2+ stores (ER) somehow gate Ca2+ entry of external Ca2+ to replenish the deficit. The physiological hallmark of the store-operated Ca2+ entry process is a large receptor-mediated transient [Ca2+]i increase followed by a prolonged high [Ca2+]i plateau phase, dependent on [Ca2+]o. A very specific and highly Ca2+-selective current (Ca2+ release activated current; ICRAC) is activated by a variety of store depletion protocols in whole-cell recordings from single blood cells (Hoth and Penner, 1992; Lewis, 1999), but store-operated entry may not be solely through ICRAC channels. From the start, TRPs have been the major suspects for the store-operated channel(s), including ICRAC. At odds with this supposition is the high Ca2+ selectivity of ICRAC compared with the cationic nonselectivity of most of the TRP family.
Here we focus on the mammalian genes and use a nomenclature adopted by a number of workers in the field (Montell et al., 2002). TRPCx, TRPVx, and TRPMx correspond to short (C, canonical), (osm-9–like, Vanilloid) and long (Melastatin family), respectively.
TRPC (Short, Canonical TRPC) Family
The TRPC group can be divided into four subgroups (TRPC1; TRPC4,5; TRPC3,6,7; TRPC2) by sequence homology as well as functional similarities. TRPC1 was the first member of the mammalian TRP family reported to form an ion channel (Zhu et al., 1996; Zitt et al., 1996). Given the widespread expression of TRPC1 and its ability to coassemble with other TRPC subunits (Xu et al., 1997; Lintschinger et al., 2000; Strubing et al., 2001), TRPC1 might be a component of different heteromeric TRP complexes. Whether TRPC1 can form functional channels in the absence of other TRP subunits is not established.
The second TRPC subfamily most closely related to TRPC1 is comprised of TRPC4 and TRPC5. Murine TRPC4 and TRPC5 can form homomeric cation channels that are activated following stimulation of Gq-coupled receptors (Okada et al., 1998; Schaefer et al., 2000) as well as receptor tyrosine kinases (Schaefer et al., 2000). Coexpression of TRPC1 and TRPC4 or TRPC5 resulted in a novel nonselective cation channel with a voltage dependence similar to N-methyl-d-aspartate receptor channels, but unlike that of any reported TRP channel (Strubing et al., 2001). The details of the activation mechanism remain elusive but the two primary products of PLC enzyme activity, IP3 and diacylglycerol, did not activate TRPC4 and TRPC5 (Hofmann et al., 1999; Schaefer et al., 2000). Both TRPC4 and TRPC5 contain a C-terminal PDZ-binding motif (VTTRL) not present in other TRPs. PDZ domain scaffolding proteins such as the Na+/H+ exchanger regulatory factor (NHERF) as well as signaling molecules like PLCβ1, coimmunoprecipitate with TRPC4 and TRPC5 (Tang et al., 2000), indicating that the channels may be part of multimolecular signaling complexes similar to the signalplex or transducisome of Drosophila photoreceptors.
TRPC3, TRPC6, and TRPC7 are ∼75% identical and when expressed constitute a cation nonselective current that rectifies in both the inward (negative voltages) and outward (positive voltages) directions. Similar to TRPC3, TRPC6 and TRPC7 are inwardly and outwardly rectifying, have relatively low selectivity of Ca2+ over Na+, are sensitive to intracellular Ca2+, and are activated by diacylglycerol (Hofmann et al., 1999; Okada et al., 1999). Their relatively high expression levels in smooth and heart muscle cells make them promising candidates for the as yet molecularly undefined nonselective cation channels in these muscle cells. In support of this idea is the finding that TRPC6 is an essential part of the α1-adrenoreceptor-activated cation channel in rabbit portal vein myocytes (Inoue et al., 2001).
Less information is available about TRPC2, which shares about 30% sequence identity with the TRPC3, TRPC6, and TRPC7 subfamilies. Full-length TRPC2 mRNA and several N-terminal splice variants have been found in mouse and rat tissue, but TRPC2 is a pseudogene in humans (Wes et al., 1995; Liman et al., 1999; Vannier et al., 1999; Hofmann et al., 2000). TRPC2 protein was localized to neuronal micovilli in rat vomeronasal organ (Liman et al., 1999). TRPC2-deficient mice display abnormal mating behavior, consistent with a role for this channel in pheromone signaling (Stowers et al., 2002).
TRPV (osm-9-Like or Vanilloid Receptor TRP) Family
Currently the TRPV family has six members grouped into three subfamilies. TRPV1 and TRPV2 are the vanilloid receptors and vanilloid-like receptors, VR-1 and VRL-1, respectively. TRPV4 is the osm-9-like OTRPC4, and TRPV5 and TRPV6 are the Ca2+-selective channels, ECaC1/CaT2 (Epithelial Calcium Channel/Calcium Transporter) and ECaC2 (also called CaT1).
The vanilloid receptors are the most well understood ion channels in this class (Caterina and Julius, 2001). VR-1 (TRPV1) is activated by the “hot” pepper-derived vanilloid compound capsaicin (Caterina et al., 1997) but is not activated by store depletion. The expressed capsaicin receptor is a relatively Ca2+-selective ion channel with an outwardly rectifying I–V relation and exhibits Ca2+-dependent desensitization. Endogenous cannabinoids receptor ligands, such as anandamide, are potential TRPV1 agonists. The exact mechanism of TRPV1 activation is not completely understood, but it is sensitive to heat (>43°C), but the temperature at which it is activated is modulated by PIP2 (Prescott and Julius, 2003). The size of the current is increased by acid pH and is modulated by intracellular PIP2, which appears to inhibit the channel (Chuang et al., 2001). Experiments with TRPV1-/- mice confirm a role for TRPV1 in transducing the nociceptive, inflammatory, and hypothermic effects of vanilloid compounds and that it contributes to acute thermal nociception and hyperalgesia following tissue injury (Caterina et al., 2000). Analysis of TRPV1-/- mice indicates that TRPV1 is essential for normal mechanically evoked purinergic signaling by the bladder urothelium (Birder et al., 2002).
The vanilloid receptor-like channel (VRL-1, TRPV2) is 50% identical to TRPV1, but is insensitive to capsaicin (Caterina et al., 1999). Like TRPV1 it is more permeable to Ca2+ than Na+ (PCa/PNa = 3/1) and is outwardly rectifying. It has been proposed to mediate high threshold (>52°C) noxious heat sensation, perhaps in the lightly myelinated Aδ nociceptors, but its presence in nonsensory tissue suggests other functions as well.
TRPV3 and TRPV4 are moderately Ca2+-selective channels. Both are sensitive to warmth in the range >31°C (Peier et al., 2002b; Smith et al., 2002; Xu et al., 2002) and >25°C, respectively. TRPV3 is also highly expressed in keratinocytes (Peier et al., 2002b), hair follicles, and on the surface of the primate tongue (Xu et al., 2002). Temperature increases TRPV4-mediated current approximately in the >25°C range, and this is potentiated by hypotonicity (Guler et al., 2002). TRPV4 current is increased by cell swelling (Liedtke et al., 2000; Strotmann et al., 2000). TRPV4-/- mice display an increase in antidiuretic hormone secretion in response to hyperosmolarity (Mizuno et al., 2003). Hypotonicity increases TRPV4 current in primary afferent nociceptive nerve fibers and is enhanced by the hyperalgesic inflammatory mediator, prostaglandin E2 (Alessandri-Haber et al., 2003).
TRPV5 (ECaC, CaT2) (Hoenderop et al., 1999) is only 30% identical to TRPV1 but is similar to TRPV6 (66% identical) and indeed many of its electrophysiological properties are indistinguishable from it. The expressed channel is strongly inwardly rectified and is relatively highly Ca2+-selective (PCa/PNa > 100) (Nilius et al., 2000; Vennekens et al., 2000). These properties are consistent with proposed mechanisms for Ca2+-selective channels in which negatively charged glutamic or aspartic acid residues provide a binding site for divalents within the pore (Tsien et al., 1987). Store-dependent activation of this channel has not been reported. TRPV6 has a wide tissue distribution. TRPV6 is Ca2+-selective (PCa/PNa >100), activated by low levels of intracellular [Ca2+], and inactivated by higher [Ca2+]I (Yue et al., 2001). Like TRPV5, TRPV6 displays a steeply inwardly rectifying I–V relation, passing most of its current at hyperpolarized potentials. It exhibits less Ca2+ selectivity and has a larger single channel conductance than ICRAC and thus is unlikely to be CRAC. Neither channel appears to be operated by store depletion.
TRPM (Long TRPC, Melastatin) Family
The TRPM (long TRP, melastatin) family has eight members divided into four groups. TRPM1 (melastatin) was initially identified through a screen of human melanoma-correlated mRNAs (Duncan et al., 1998). Although analysis of TRPM1 mRNA indicates wide expression, TRPM1's electrophysiological properties have not been studied.
TRPM2 (Nagamine et al., 1998) is a Ca2+-permeant nonselective channel with a linear I–V relation (Perraud et al., 2001). ADP-ribose and potentially NAD (Perraud et al., 2001; Sano et al., 2001) bind a C-terminal NUDT9 Nudix hydrolase family domain to gate the channel. As for many Ca2+-permeant channels, it appears to be inactivated by [Ca2+]i. TRPM3 (Grimm et al., 2003; Lee et al., 2003) is a Ca2+-permeant nonselective channel with a linear I–V relation that is constitutively active when heterologously expressed. Its activity is increased by hypotonicity (200 mOsm/l) (Grimm et al., 2003), and its expression pattern indicates that TRPM4 functions in kidney and in the central nervous system.
TRPM4 and TRPM5 are the only monovalent-selective ion channels of the TRP family. Both are Ca2+-activated ∼20 to 30 pS nonselective channels (Launay et al., 2002; Hofmann et al., 2003). G protein receptors coupled to PLC-dependent endoplasmic reticular Ca2+ release activate these channels, perhaps by direct Ca2+ binding (Launay et al., 2002; Hofmann et al., 2003). Although their instantaneous I–Vs are linear, they exhibit voltage dependence with continued depolarization (Hofmann et al., 2003; Nilius et al., 2003). TRPM5 is activated by sweet, umami, and bitter taste G protein-coupled receptor pathways (Zhang et al., 2003).
TRPM6 and TRPM7 contain a functional kinase domain within their polypeptide chain. TRPM7 was identified in a yeast two-hybrid screen as a protein interacting with PLCβ1, and was the first member of the TRPM group to be expressed as a functional ion channel (Clapham et al., 2001; Runnels et al., 2001). TRPM7 passes little inward current under physiological conditions, is permeant to both Ca2+ and Mg2+, and is inhibited by ∼0.6 mM intracellular-free Mg2+ (Nadler et al., 2001). TRPM7 current increases slowly under whole-cell recording conditions, and it is inactivated by PIP2 hydrolysis by PLCβ or PLCγ (Runnels et al., 2002). The function of the kinase domain is poorly understood but is not required for channel activation (Runnels et al., 2002; Schmitz et al., 2003). The substrates of the kinase, an atypical serine/threonine kinase, have not been identified (Yamaguchi et al., 2001). Familial hypomagnesemia with secondary hypocalcemia is caused by a mutation in TRPM6 (Schlingmann et al., 2002; Walder et al., 2002).
TRPM8 is up-regulated in prostate and other cancers (Tsavaler et al., 2001). TRPM8 is a nonselective, outwardly rectifying channel that can be activated by cold (8–28°C) and enhanced by “cooling” compounds such as menthol and icilin (McKemy et al., 2002; Peier et al., 2002a). It is therefore likely to contribute to the sensation of cold temperature.
TRPA Ankyrin-Repeat TRP Channel Family
ANKTM1 is a Ca2+-permeant nonselective channel with ∼14 ankyrin repeats in its N terminus. It is activated by noxious cold (Story et al., 2003). TRPA1 is found in nociceptive sensory DRG, and its homolog in Drosophila, painless. Many other TRP channels have not been systematically tested for temperature sensitivity, but such a comparison would clarify the field.
Conclusion
The TRP channels are a family of six transmembrane-spanning domain proteins expressed in low numbers per cell to yield small net inward currents. At this time, there is no unifying theme in their function or mechanism for activation. The TRPV (osm-9 like/vanilloid) subfamily is the most well characterized of the group and includes ion channels that are certainly involved in neuronal pain pathways, perhaps to sense heat and osmolarity. The TRPM (long TRPC, melastatin) subfamily may well be the most novel, with potential roles in Ca2+-dependent signaling, control of cell cycle progression, division or migration, and thermosensation. TRP proteins are common in many cell types, making expression of confirmed monomeric channels difficult. Several TRPs are known to form heteromultimers, and their electrophysiological properties depend on the subunit composition. The multipotent phosphatidylinositol pathway is involved in most TRP regulation, but the details of this regulation are just beginning to be elucidated.
Footnotes
-
↵1 This work was previously published in Catterall WA, Chandy KG, and Gutman GA, eds. (2002) The IUPHAR Compendium of Voltage-Gated Ion Channels, International Union of Pharmacology Media, Leeds, UK.
-
↵2 Abbreviations: TRP, transient receptor potential; Ca2+, calcium; CaT, calcium transporter; ER, endoplasmic reticulum; ECaC, epithelial calcium channel transporter; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; KcsA, prokaryotic potassium-selective channel; PCa/PNa, ratio of calcium permeability to sodium permeability; PKD, polycystic kidney disease; PIP2, phosphatidylinositol 4,5-bisphospate; PLC, phospholipase C; S, segment, TM, transmembrane; TRPA, ankyrin-repeat TRP; TRPC, canonical TRP; TRPM; melastatin TRP; TRPV, vanilloid TRP.
-
DOI: 10.1124/pr.55.4.6.
- The American Society for Pharmacology and Experimental Therapeutics