Visual Overview
Abstract
Systemic diseases of liver origin (SDLO) are complex diseases in multiple organ systems, such as cardiovascular, musculoskeletal, endocrine, renal, respiratory, and sensory organ systems, caused by irregular liver metabolism and production of functional factors. Examples of such diseases discussed in this article include primary hyperoxaluria, familial hypercholesterolemia, acute hepatic porphyria, hereditary transthyretin amyloidosis, hemophilia, atherosclerotic cardiovascular diseases, α-1 antitrypsin deficiency-associated liver disease, and complement-mediated diseases. Nucleic acid therapeutics use nucleic acids and related compounds as therapeutic agents to alter gene expression for therapeutic purposes. The two most promising, fastest-growing classes of nucleic acid therapeutics are antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs). For each listed SDLO disease, this article discusses epidemiology, symptoms, genetic causes, current treatment options, and advantages and disadvantages of nucleic acid therapeutics by either ASO or siRNA drugs approved or under development. Furthermore, challenges and future perspectives on adverse drug reactions and toxicity of ASO and siRNA drugs for the treatment of SDLO diseases are also discussed. In summary, this review article will highlight the clinical advantages of nucleic acid therapeutics in targeting the liver for the treatment of SDLO diseases.
Significance Statement Systemic diseases of liver origin (SDLO) contain rare and common complex diseases caused by irregular functions of the liver. Nucleic acid therapeutics have shown promising clinical advantages to treat SDLO. This article aims to provide the most updated information on targeting the liver with antisense oligonucleotides and small interfering RNA drugs. The generated knowledge may stimulate further investigations in this growing field of new therapeutic entities for the treatment of SDLO, which currently have no or limited options for treatment.
I. Introduction
A. Systemic Diseases of Liver Origin
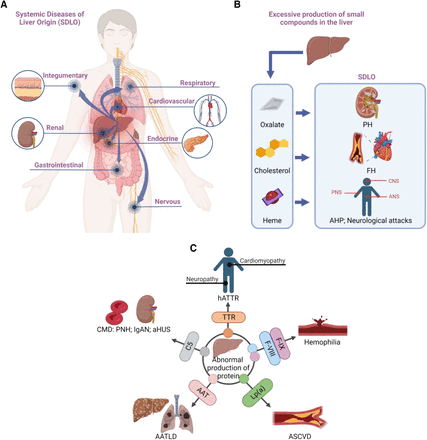
Systemic diseases of liver origin (SDLO) refers to diseases diagnosed in other organ or tissue systems throughout the body due to abnormal liver functions with or without etiology of liver injury or disease (Shimizu, 2008; Edwards and Wanless, 2013). Normally, SDLO is caused by irregular production of either small molecule compounds, such as carbohydrates, lipids, amino acids, and vitamins, or large molecule compounds, for example nucleic acids and proteins in the liver. The irregularly produced compounds are released from the liver into blood and transported and deposited to other organs or tissue systems, including but not limited to nervous, cardiovascular, endocrine, respiratory, gastrointestinal, integumentary, and renal systems, causing systemic diseases (Fig. 1A) (Wang et al., 2021).
Systemic diseases of liver origin (SDLO) and its associated irregular productions in the liver. (A) SDLO are diseases diagnosed in other organs due to abnormal liver productions. The irregularly produced compounds are released from the liver and deposited to other organs or tissue systems, including but not limited to nervous, cardiovascular, endocrine, respiratory, gastrointestinal, integumentary, and renal systems, causing systemic diseases. (B) Irregular productions of small chemical compounds in the liver are associated with SDLO. PH is caused by excessive production of oxalate in the liver and overaccumulation in kidneys and urine to form kidney stones and damage. FH is caused by genetic defects in the LDL-R for irregular production of cholesterol in the liver and overaccumulation in blood to form plaques and lead to coronary artery disease. AHP is caused by irregular production of heme in the liver and overaccumulation in blood with neurologic attacks, resulting in nerve pain, vomiting, neuropathy, and seizures. (C) Irregular productions of proteins in the liver are associated with SDLO. TTR hATTR is caused by expression of an abnormal TTR protein in the liver and over-deposit of the abnormal TTR as amyloid in various organs and peripheral nerves in the body with cardiomyopathy or neuropathy. Hemophilia is caused by production of mutant coagulation factors F-VIII and F-IX proteins in the liver, resulting in X-linked bleeding disorders. ASCVD is caused by Lp(a) in the liver and overaccumulation in arteries with a high risk of heart attack and strokes; AATLD is caused by irregular production of AAT with wrong shape in the liver and deficient in lung with a high risk of developing hepatocellular carcinoma and lung diseases. CMD, including PNH, aHUS, and IgAN, is caused by hyperactivation of C5 protein in the liver and over deposit in blood and the kidneys, causing inflammation that damages red blood cells and kidney tissues. ANS, autonomic nervous system; CNS, central nervous system; PNS, peripheral nervous system.
Some examples of SDLO are associated with irregular production of small chemical compounds in the liver (Fig. 1B): 1) primary hyperoxaluria (PH), including PH type 1 (PH1), 2 (PH2), and 3 (PH3), caused by excessive production of oxalate in the liver and overaccumulation in kidneys and urine to form kidney stones and damage (Bhasin et al., 2015; Witting et al., 2021); 2) familial hypercholesterolemia (FH) caused by genetic defects in the low-density lipoprotein (LDL) receptor (LDL-R) for irregular production of cholesterol in the liver and overaccumulation in blood to form plaques, leading to coronary artery disease (Benito-Vicente et al., 2018); and 3) acute hepatic porphyria (AHP) caused by irregular production of heme in the liver and overaccumulation in blood with adverse neurologic targeting, resulting in nerve pain, vomiting, neuropathy, and seizures (Oliveira Santos and Leal Rato, 2021; Kothadia et al., 2022).
The examples of SDLO associated with irregular production of proteins in the liver (Fig. 1C) include 1) hereditary transthyretin (TTR) amyloidosis (hATTR) caused by expression of an abnormal TTR protein in the liver and over deposit of the abnormal TTR as amyloid in various organs and peripheral nerves in the body with cardiomyopathy or neuropathy (Sekijima, 1993); 2) hemophilia caused by productions of mutant coagulation factor VIII (F-VIII) and factor IX (F-IX) proteins in the liver, resulting in X-linked bleeding disorders (Bowen, 2002; Castaman and Matino, 2019); 3) atherosclerotic cardiovascular diseases (ASCVDs) caused by excessive production of apolipoprotein a [Lp(a)] in the liver and overaccumulation in arteries with a high risk of heart attack and strokes (Park and Oh, 2019; Tada et al., 2019); 4) α-1 antitrypsin deficiency-associated liver disease (AATLD) caused by irregular production of α-1 antitrypsin with wrong shape in the liver and deficient in lung with a high risk of developing hepatocellular carcinoma and lung diseases (Mitchell and Khan, 2017); and 5) complement-mediated diseases (CMD), including paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and immunoglobulin A nephropathy (IgAN), caused by hyperactivation of complement component 5 (C5) protein in the liver and excessive deposit in blood and the kidneys, causing inflammation that damages red blood cells and kidney tissues (Lafayette and Kelepouris, 2018; Rizk et al., 2019; Knoppova et al., 2021; Tanaka et al., 2021; Dingli et al., 2022).
Current treatment options for SDLO mainly focus on symptom control through traditional small chemical molecule pharmacotherapies or nonpharmacologic therapies. Some of these therapies may lack specificity, may not target the disease origin of the liver, and have not been efficacious in controlling symptoms and managing disease states (Hoppe and Martin-Higueras, 2022). Some of those small molecule drugs act on downstream products in a pathway, whereas the need for therapy in many of these SDLO should target upstream at the sites of the disease origin. Numerous SDLO diseases currently have no therapeutic options approved to cure the diseases, such as hemophilia and IgAN. As the prevalence of SDLO continues to increase, the need for better therapies becomes more imperative. Nucleic acid therapeutics is an area of pharmacology that can potentially achieve positive implications in the therapeutic management of patients with SDLO (Zogg et al., 2022).
B. Nucleic Acid Therapeutics
Nucleic acid therapeutics is an area of drug discovery that has been rapidly growing in the last decade and is predicted to continue growing and developing in the next decades (Kulkarni et al., 2021; Yu and Tu, 2022). The two fastest-growing areas of nucleic acid therapeutics are antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) (Bajan and Hutvagner, 2020). In the last decade, the US Food and Drug Administration (FDA) has approved 11 ASO and five siRNA drugs (Zhang et al., 2021; Migliorati et al., 2022b), among which seven drugs target SDLO diseases (Table 1), including lumasiran for PH1 (Scott and Keam, 2021); inotersen (Keam, 2018), patisiran (Hoy, 2018), and vutrisiran (Keam, 2022) for hATTR; mipomersen (Hair et al., 2013) and inclisiran (Lamb, 2021) for FH; and givosiran for AHP (Scott, 2020). Beyond the FDA-approved ASO and siRNA drugs targeting SDLO, several dozens of ASO and siRNA therapeutic agents are currently in late clinical trial stages and may receive FDA approval in the next few years (Crooke et al., 2021; Friedrich and Aigner, 2022), some of which with SDLO disease indications (Table 1), for example nedosiran for PH1, PH2, and PH3 (Liu et al., 2022); eplontersen for hATTR (Cantone et al., 2022); fitusiran for hemophilia (Nogami and Shima, 2023); pelacarsen and olpasiran for ASCVD (Sosnowska et al., 2022); fazirsiran and belcesiran for AATLD (Strnad et al., 2022); and cemdisiran for CMD, such as PNH, aHUS, and IgAN (Badri et al., 2021).
Discovery and development of ASO and siRNA drugs for the treatment of SDLO diseases
This review aims to provide the most advanced knowledge on the epidemiology, symptoms, genetic causes, and treatment options with seven FDA-approved as well as eight potential ASO and siRNA drugs in late clinical trials for the eight listed SDLO diseases. Challenges, knowledge gaps, and future perspectives of targeting the liver with nucleic acid therapeutics for treating SDLO diseases will also be discussed.
II. Current Knowledge
A. Principle of Drug Action of Antisense Oligonucleotide and Small Interfering RNA Drugs for Nucleic Acid Therapeutics
1. Principle of Drug Action of Antisense Oligonucleotide Drugs
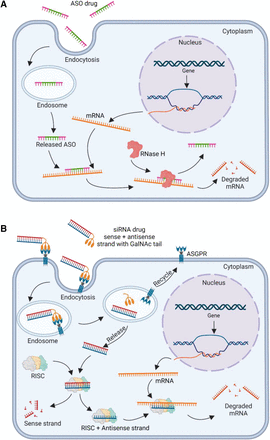
After the concept was demonstrated in 1977 that gene expression can be modified with exogenous nucleic acids by use of a single-stranded ASO to inhibit the translation of complementary RNA in a cell-free system (Paterson et al., 1977), it took nearly 20 years for drug discovery and development (Oberemok et al., 2018) to get the first ASO drug, fomivirsen, approved by the FDA in 1998 to treat cytomegalovirus retinitis in the eye (Roehr, 1998). An ASO drug is a synthetic, short (20–22 nucleotides), single-stranded DNA analog formulated in solution and administrated by intravitreal, intrathecal, or subcutaneous injection or intravenous infusion (Migliorati et al., 2022b). Gapmer ASO is more frequently incorporated in the ASO design, which contain a central sequence of DNA nucleotides “DNA gap” flanked by RNA nucleotides in both ends, showing reduced off-target effects than pure DNA ASO (Yasuhara et al., 2022). The principle of drug action (PDA) of ASO drugs relies on complementary antisense hybridization to target RNAs, inhibiting gene expression and protein production in multiple ways. One mechanism includes RNase-H endonuclease–mediated mRNA degradation, in which RNase-H endonuclease cleaves the newly formed double-stranded hybrid of ASO and target mRNA (DNA:RNA heteroduplex), leading to RNase-H–dependent degradation of the target sequence. At the same time, the ASO remains intact and can be reused (Nishina et al., 2015). All ASO drugs discussed in this article use the RNase-H–mediated degradation mechanism as their PDA for therapeutic purpose (Fig. 2A). Another mechanism of PDA is translational arrest by steric hindrance of ribosomal activity, which prevents the translation of mRNAs solely by the ASO binding (Amanat et al., 2022). ASOs can also induce exon skipping of the target pre-mRNA sequences, causing the removal of the targeted exons from the pre-mRNA and producing mRNAs with skipped exons (Matsuo, 2021). Lastly, ASO binding to the target sequence can result in the destabilization of pre-mRNA in the nucleus and targeted destruction of mRNA expression (Roberts et al., 2020). Recent developments in ASO therapeutics include the pursuit of new mechanisms in addition to RNase-H, including therapies that follow traditional base-pairing for exon skipping, splice switching, and mRNA sequestration (Frazier, 2015).
Principle of drug action of ASO and siRNA drugs. (A) ASO drugs use an RNase H–mediated mRNA degradation process. An ASO drug, such as mipomersen and inotersen with a gapmer design, gets into a cell through endocytosis and is first stored in the endosome, then undergoes an endosomal release. By complementary base pairing with the target mRNA, ASO and mRNA form a DNA:RNA heteroduplex that can be recognized by RNase H. In the heteroduplex, the mRNA is degraded while the ASO remains intact and can be reused. (B) siRNA drugs use RISC-mediated mRNA degradation process. The GalNAc-conjugated siRNA binds to ASGPR with high affinity and helps this siRNA to be taken up by endocytosis. Then, ASGPR is released by endosome and recycled on the cell membrane. At the same time, siRNA is released to load into a RISC. The antisense strand is activated by selective removal of the sense strand. The antisense strand then guides the RISC to bind to the target mRNA sequence, leading the Argonaute protein in the RISC to cleave the target mRNA sequence.
2. Principle of Drug Action of Small Interfering RNA Drugs
Since the discovery of siRNAs in Caenorhabditis elegans as a mechanism in specific biologic systems to interfere with the function of an endogenous gene in 1998 (Fire et al., 1998), siRNAs have become a powerful tool for silencing of expression of any gene in a sequence-specific manner. Later development of this Nobel Prize–winning discovery offers a unique therapeutic platform to target disease-associated genes for the treatment of rare life-threatening diseases and some common diseases (Setten et al., 2019; Wang et al., 2020). A siRNA drug is a synthetic, short (21–25 nucleotides), double-stranded RNA molecule formulated in solution and administrated by subcutaneous injection or intravenous infusion. The main mechanism of drug action of siRNA drugs includes inhibiting gene expression via RNA interference (Dana et al., 2017). A siRNA induces an RNA-interference (RNAi) pathway in the cytoplasm and nucleus (Dudley and Goldstein, 2003), utilizing the RNA-induced silencing complex (RISC). The antisense strand is activated by selective removal of the sense strand. The antisense strand then guides the RISC to bind to the target mRNA sequence, leading the Argonaute protein in RISC to cleave the target sequence (Xu et al., 2019). The degree of complementarity also plays a key role in directing RNAi machinery and leads to different types of inhibition (Aagaard and Rossi, 2007). If the siRNA perfectly complements the target sequence, mRNA cleavage and degradation are directed. All siRNA drugs discussed in this article use the mechanism of RISC-mediated perfect complement sequence match as their PDA for therapeutic purpose (Fig. 2B). If not perfectly complementary, a bulge forms that directs the RNAi machinery toward translational inhibition (Van den berg et al., 2008). This makes the RNA molecules unable to be translated into more proteins.
siRNAs have distinct sources from internal microRNAs (miRNAs). Whereas miRNAs are endogenous small RNA molecules encoded by the genome (Ha and Kim, 2014), siRNAs are custom designed and synthesized within laboratory or manufactory (Traber and Yu, 2023). Despite their disparate origins, both siRNAs and miRNAs get involved in the same posttranscriptional gene regulation pathway, RISC-mediated mRNA cleavage or degradation, and subsequent gene silencing. Generally, miRNAs bind to mRNAs with partially complementary sequences, primarily within the 3′-untranslated region (3′-UTR). This interaction leads to translational repression or mRNA degradation. Conversely, most siRNAs are in perfect complementarity with coding sequence regions of specific disease-associated mRNAs, resulting in precise mRNA cleavage and degradation (Lai et al., 2013; Yu and Tu, 2022).
3. Chemical Modifications of Antisense Oligonucleotide and Small Interfering RNA Drugs
From proof of concept to the first FDA-approved drug, the discovery and development of both ASO and siRNA drugs took nearly two decades. Less efficacy and high risk of toxicity are two main reasons for the failure of the discovery and development of ASO and siRNA drugs in the early stage (Watts and Corey, 2012). The use of chemical modifications to ASO and siRNA drugs allow these therapeutic agents to become more versatile and effective in the later development stage. Although siRNAs can work effectively without chemical modifications (specifically in direct drug administration), they are often found to cause off-target effects and to have low efficacy rates. ASO therapeutics face a similar problem without modifications, having low efficacy rates and adverse side effects. The standard modifications found in siRNA and ASO therapeutic agents are additions to the phosphate backbone and bases. Some common groups added include phosphorothioate (PS), 2’-O-methyl (2’-OMe), 2’-fluoro (2’-F), and 2’-O-methyloxyethyl (2’-MOE) groups. These specific modifications to siRNA and ASO therapeutics allow them to be resistant to nuclease degradation (Chen et al., 2019; Gangopadhyay and Gore, 2022) and to reduce nonspecific effects (Yoo et al., 2004). Another modification used in the FDA-approved ASO drugs of eteplirsen, golodirsen, viltolarsen, and casimersen for treating Duchenne muscular dystrophy is the phosphorodiamidate morpholino oligomer, which uses a six-membered morpholino ring to replace the five-membered rings in DNA and RNA oligonucleotides (Migliorati et al., 2022b). As uncharged nucleic acid analogs, phosphorodiamidate morpholino oligomer nucleotides are more resistant to a variety of enzymes present in biologic fluids, making them highly stable and effective through a variety of administration routes (Warren et al., 2012; Nan and Zhang, 2018). Although not used in the FDA-approved ASO and siRNA drugs, peptide nucleic acids may become a promising approach in future ASO and siRNA drug design. Peptide nucleic acids are synthetic analogs of DNA with peptide backbone connected to nucleobases via a linker, making them to have unique properties of resistance to enzymatic digestion, higher biostability, and great hybridization affinity toward DNA and RNA (Montazersaheb et al., 2018).
4. Liver-Specific Delivery of Antisense Oligonucleotide and Small Interfering RNA Drugs
The failure of discovery and development of ASO and siRNA drugs in the early stage is also related to the need of formulating efficient delivery systems. Discovery of N-acetylgalactosamine (GalNAc) for liver-specific targeting has significantly sped up the discovery phases for ASO and siRNA drugs targeting SDLO diseases (Springer and Dowdy, 2018; Debacker et al., 2020; Cui et al., 2021). GalNAc is used in both ASOs and siRNAs to make delivery more efficient. GalNAc binds to asialoglycoprotein receptor (ASGPR) with high affinity and is taken up by endocytosis in hepatocytes (Nair et al., 2014). Then, the conjugate dissociates from the receptor, leading to the uptake of the oligonucleotides into the cytoplasm, while the sugars and branches of GalNAc are lysed. In addition, ASGPR is highly expressed only in hepatocytes, giving some natural selectivity (Shi et al., 2013). One study elucidated preferential targeting of GalNAc-siRNAs to hepatocytes, with limited distribution to nonparenchymal liver cells and marginal presence in other tissues, albeit exhibiting some interspecies variations (Janas et al., 2018). Specifically, in rats subjected to repeated doses of ≥30 mg/kg, GalNAc-siRNA accumulation occurred in proximal renal tubular cells, characterized by distinct punctate cytoplasmic basophilic granules upon hematoxylin and eosin staining. Conversely, in monkeys exposed to toxicologic doses ≥100-fold higher than standard pharmacologic doses, drug accumulation was prominently observed as basophilic granules within hepatic Kupffer cells and, to a lesser extent, within hepatocytes.
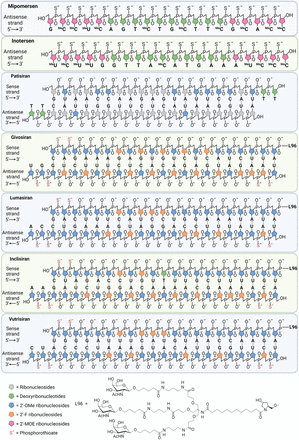
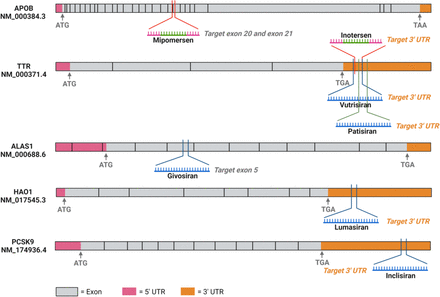
Lipid nanoparticles (LNPs) may also deliver ASOs and siRNAs to cells through the membrane more easily (Leung et al., 2014). The first FDA-approved siRNA drug, patisiran, is delivered by LNPs to the liver to treat hATTR (Zhang et al., 2020b). Figure 3 shows the chemical modifications and delivery systems for the seven FDA-approved ASO and siRNA drugs targeting the discussed SDLO diseases. The binding positions of the ASO and siRNA drugs on their targeting mRNAs are shown in Fig. 4, indicating most of them (patisiran, vutrisiran, lumasiran, and inclisiran) targeting the 3′-UTR regions, with the exception of mipomersen and givosiran in one or two translation exons. This observation highlights the significance of selecting an appropriate target region for siRNA design.
Chemical modifications and delivery systems for the seven FDA-approved ASO and siRNA drugs. Mipomersen and inotersen are ASO drugs with 20 nucleotides. They share the exact same chemical modifications beyond the phosphate backbone and bases. Five 2’-MOE moieties are added at the 5′ and 3′ ends and phosphorothioate (S-) modifications throughout the backbone. Substitution at the 5-position of the cytosine (C) and uracil (U) bases with a methyl group is indicated by Me. Patisiran is the first siRNA drug approved by FDA with 11 2’-OMe–modified sugar residues in between and two 2’-deoxythymidine residues added to the 3′ ends of both strands. Givosiran is chemically modified with 28 2’-OMe and 16 2’-F groups in the ribose sugar moieties, and two S- modifications are added at the 5′ end of the sense strand as well as both ends of the antisense strand. Lumasiran has 34 2’-OMe and 10 2’-F groups in the ribose sugar moieties, and two S- modifications are added at the 5′ end of the sense strand as well as both ends of the antisense strand. Inclisiran has been chemically modified with 31 2’-OMe and 12 2’-F groups in the ribose sugar moieties, and two S- modifications are added at the 5′ end of the sense strand and both ends of the antisense strand. Notably, there is a deoxyribonucleotide in the middle of its sense strand. Vutrisiran has 35 2’-OMe and nine 2’-F groups in the ribose sugar moieties, and two S- modifications are added at the 5′ end of the sense strand as well as both ends of the antisense strand. Patisiran is encapsulated in an LNP for optimum circulation time and efficient delivery into cells. Unlike patisiran, the other four approved siRNA drugs are all connected with a GalNAc (L96) at the end of 3′ ends of their sense strands, increasing potency, stability, and uptake into hepatocyte cells.
Binding positions of the FDA-approved ASO and siRNA drugs on their targeting mRNAs. Mipomersen is designed to selectively interact with the conjoined region of exon 20 and exon 21 within the mRNA sequence of APOB, encompassing one nucleotide from exon 20 and 19 nucleotides from exon 21. In contrast, Inotersen exerts its mechanism by directly binding to the 3′-UTR of the TTR mRNA. Notably, both Inotersen and vutrisiran share a common targeting site excepting the presence of an additional nucleotide at the 3′ terminus of vutrisiran. Patisiran likewise directs its therapeutic effect toward TTR, with a partially overlapping sequence section shared with both inotersen and vutrisiran. Givosiran binds to exon 5 of the 5′- ALAS1 mRNA. Lumasiran and inclisiran elicit their effects by interacting with the 3′-UTR regions of their respective target mRNAs, HAO1 and PCSK9, each within their distinct contexts.
Although small molecule therapeutics have carried us far in pharmacotherapy, there are still undruggable targets where small molecules cannot be used as therapy. An example of “undruggable” targets are enzymes. Although small molecules can target some enzymes, for others it is simply not possible to target them. An example of these non–pharmacotherapy-targeted enzymes is Ras proteins, which are GTPases (Lu et al., 2016). There are also time difficulties in developing small molecule pharmacotherapy. Small molecule therapeutics take longer to develop than ASO and siRNA therapeutics. ASO and siRNA therapeutics provide an alternative method of therapy. Whereas small molecule therapeutics typically target proteins, nucleic acid therapeutics target RNAs. This allows longer-lasting effects to be seen, which are impossible through targeting proteins directly. Nucleic acid therapeutics like ASOs and siRNAs have a highly promising future in pharmacotherapy. This is seen in the ASO and siRNA therapeutics that target SDLO diseases.
B. Treatment of the Systemic Diseases of Liver Origin Diseases with Antisense Oligonucleotide and Small Interfering RNA Drugs
1. Targeting the Liver with the Small Interfering RNA Drug Lumasiran and a Potential Small Interfering RNA Drug, Nedosiran, for the Treatment of Primary Hyperoxaluria
a. Epidemiology of primary hyperoxaluria, including primary hyperoxaluria 1, primary hyperoxaluria 2, and primary hyperoxaluria 3
PH is a rare genetic disorder of overproduction of oxalate in the liver, resulting in long-term accumulation of oxalate and urea in kidneys, subsequently producing chronic kidney disease and renal failure. This genetically inherited disorder is rare, with an incidence rate of approximately 1 to 2 cases per 100,000 in the US population (Shah et al., 2023) and 1–3 cases per 100,000 in the European populations (Cochat et al., 1995; Kopp and Leumann, 1995; van Woerden et al., 2003). It is also reported to be more predominant in countries where consanguineous marriages are common, particularly in regions of Northern Africa and the Middle East (Soliman et al., 2022). There are 3 types of PH: PH1, PH2, and PH3. PH1 is the most common and rapidly progressing disorder of the three, accounting for the majority (up to 80%) of the reported PH cases (Bhasin et al., 2015).
b. Symptoms of primary hyperoxaluria
PH results in nephrolithiasis, urolithiasis, and high levels of oxalate in the kidneys. This chronic kidney disease is the outcome of renal calcium oxalate accumulation, ultimately leading to frequent stone formation and kidney failure (Ermer et al., 2016). As kidney failure progresses, the accumulation of oxalate may spread to other organ systems. Although symptoms may appear at any age, typically they tend to appear in childhood. Just like many other diseases, symptoms and progression of PH may differ from patient to patient. Symptoms associated with PH1 include failure to thrive, oxalate deposits in the kidney, formation of kidney stones in the urinary tract, dysuria, hematuria, and frequent urinary tract infections (Ermer et al., 2016). PH1 usually develops during childhood and progresses during adulthood, leading to chronic kidney disease and end-stage renal failure. PH2 also usually develops during childhood, but it presents itself with milder symptoms, accounting for less than 10% of all PH cases (Ermer et al., 2016). PH2 can also progress to cause kidney failure, although that occurs much later in life as compared with the rapid progression of PH1. Symptoms of PH3 also begin in childhood and can be like those who have PH1 or PH2; however, they are much milder. Due to the rareness of the PH3 disorder, not much can be concluded about its progression. The occurrence of kidney failure and nephrocalcinosis is very rare, with less prevalence of kidney and urinary stones in patients who have PH3 (Singh et al., 2022).
c. Genetic causes of primary hyperoxaluria 1, primary hyperoxaluria 2, and primary hyperoxaluria 3
These disorders result from defects in various enzymes that are involved in the metabolism of glyoxylate. Oxalate is an end product of the metabolism of some diet-derived nutrients in the liver, which is normally excreted from the body via the kidneys. The small amounts of oxalate that the liver generates under normal conditions can be successfully eliminated by the kidneys. However, when the liver produces excessive oxalate, this results in its accumulation in the kidneys leading to nephrocalcinosis and kidney failure (Ermer et al., 2016). This occurs due to defects of certain enzymes in the liver that are responsible for regulating the production and excretion of oxalate.
PH1 is caused by genetic alterations in the alanine-glyoxylate aminotransferase (AGXT) gene, which leads to a deficiency in the expression of this enzyme (Tarn et al., 1997). When present at normal levels, this enzyme converts glyoxylate to glycine. The absence of the AGXT enzyme leads to accumulation of glyoxylate and overproduction of oxalate by lactate dehydrogenase (Tarn et al., 1997). Glyoxylate can also be reduced to glycolate using NADH or NADPH by the enzyme glyoxylate reductase (GR). PH2 results from a mutation of the glyoxylate reductase-hydroxypyruvate reductase (GRHPR) gene, which encodes the enzyme GR (Cregeen et al., 2003). The lack of GR can contribute to further increase in the levels of oxalate in cytosol. PH3 results from a defect in the 4-hydroxy-2-oxoglutarate aldolase (HOGA1) gene (M’dimegh et al., 2017). A deficiency of this gene leads to a loss of production of its enzyme HOGA1, which favors the conversion of 4-hydroxy-2-oxoglutarate (HOG) to pyruvate and glyoxylate. The exact mechanism of how a deficiency in the HOGA1 gene causes accumulation of oxalate resulting in PH3 remains unclear (Bar et al., 2021).
d. Current treatment options of primary hyperoxalurias
Changes to low-salt and restricted diets in oxalates may help to lower the levels of oxalate in urine (Mitchell et al., 2019). Calcium supplements are the primary medical treatment of PHs when they are taken with meals (Takei et al., 1998). Interaction of oxalate with calcium in the gut could limit the intestinal absorption of oxalate and promote its fecal excretion. Prescription of vitamin B-6 can help reduce oxalate in the urine in some PH patients (Hoyer-Kuhn et al., 2014). Other medications, which may help to reduce oxalate in the body, include thiazide diuretics (Vigen et al., 2011) and phosphates and citrate (Krieger et al., 2015). However, these medications can only slow down the progression of PHs, not cure the disease.
When kidney failure caused by kidney stones in PH patients becomes severe, dialysis and kidney transplantation can be performed to treat PH, however, kidney stones may reappear post transplantation due to dysfunction of the liver that consistently produces excessive oxalate (Cornell et al., 2022). Simultaneous kidney and liver transplantation may be the only treatment that might cure some types of PHs (Cornell et al., 2022) until the recent development on siRNA therapeutics.
e. Treatment of primary hyperoxaluria 1 with the small interfering RNA drug lumasiran and treatment of PH1, PH2, and PH3 with a potential small interfering RNA drug, nedosiran
In November 2020, The FDA approved lumasiran as the first medication possible to cure PH1 disease for pediatric and adult patients (Scott and Keam, 2021). Lumasiran (brand name Oxlumo) is a siRNA drug developed by Alnylam Pharmaceuticals with a double-stranded chemical-modified ribonucleic acid conjugated to GalNAc for liver-specific delivery (sequence and chemical modifications are shown in Fig. 3). By targeting on the 3′-UTR region of hydroxy acid oxidase 1 (HAO1) mRNA (Fig. 4), lumasiran works by reducing the HAO1 mRNA, leading to lower levels of glycolate oxidase (GO) enzyme in hepatocytes (D’Ambrosio and Ferraro, 2022). The reduced GO enzyme results in increased levels of glycolate and decreased levels of oxalate in the liver, kidneys, and urine. Lumasiran is administered subcutaneously with three loading doses (6 mg/kg) once monthly and maintenance doses (3-6 mg/kg) once every 3 months.
As shown in Table 1, the pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of lumasiran were determined in a single blind, placebo-controlled phase 1/II study (NCT02706886) with 32 healthy and 20 pediatric and adult patients with PH1. The study concluded that lumasiran had an acceptable safety profile with no serious adverse drug reactions (ADRs), and all patients with PH1 had reduced urinary oxalate excretion to near-normal levels (Frishberg et al., 2021). The efficacy of lumasiran was further confirmed in a randomized, double blind, placebo-controlled phase 2 study (NCT05161936) using adult patients with recurrent oxalate kidney stones and elevated urinary oxalate levels.
Lumasiran earned the FDA’s approval due to the positive results found in several phase 3 studies, including ILLUMINATE-A NCT03681184 (Garrelfs et al., 2021), ILLUMINATE-B NCT03905694 (Sas et al., 2022; Hayes et al., 2023), and ILLUMINATE-C NCT04152200 (Michael et al., 2023). These studies further verified that lumasiran resulted in substantial reductions in plasma and urine oxalate levels with acceptable safety in patients with PH1 who have advanced kidney disease. With only 2 and half years on the market, it may be too soon to have real-world studies to evaluate the PK, PD, and safety of lumasiran in the treatment of PH1, but lumasiran has been considered as a turning point in the management of PH1 (D’Ambrosio and Ferraro, 2022).
Due to targeting HAO1 mRNA and GO enzyme, lumasiran is only effective in the treatment of PH1. Pharmacologic options are not available yet for the treatment of PH2 and PH3. The situation may be changed in the near future with the development of a candidate siRNA drug, nedosiran, for the treatment of all types of PH (Liu et al., 2022).
Under development by Dicerna Pharmaceuticals, nedosiran is a candidate siRNA drug with chemical modifications and GalNAc conjugation. By targeting the lactate dehydrogenase mRNA, nedosiran could reduce levels of lactate dehydrogenase enzyme, a key enzyme mediating the final step of the production of oxalate from glyoxylate in the liver, ultimately resulting in reducing the formation of oxalate in all three types of PH.
As shown in Table 1, the PK, PD, safety, and tolerability of nedosiran have been assessed and will be continually determined through various clinical studies from phase 1 to 3, including PHYOX1 (NCT03392896), a phase 1 study with healthy volunteers and PH patients; PHYOX2 (NCT03847909), a pivotal randomized phase 2 study with PH1 and PH2 patients (Baum et al., 2023); PHYOX4 (NCT0455486), a phase 1 study with PH3 patients (Goldfarb et al., 2023); PHYOX7 (NCT04580420), a phase 2 study with patients with PH1/2 and end-stage renal disease; PHYOX8 (NCT05001269), a phase 2 study with pediatric patients with PH and relatively intact renal function; and PHYOX3 (NCT04042402), a long-term extension phase 3 study with patients with PH1, PH2, and PH3. In the PHYOX2 study, the PH1 subgroup maintained a sustained reduction of urine oxalate levels, whereas no consistent effect was seen in the PH2 subgroup (Baum et al., 2023). In the PHYOX4 study, nedosiran was well tolerated without safety concerns, and a trend toward urinal oxalate lowering was observed in PH3 patients with nedosiran (Goldfarb et al., 2023). The results from the other ongoing clinical studies have not been released yet. Whether nedosiran can treat all types of PH needs to be tested in more PH2 and PH3 patients, pediatric patients, and patients with renal impairment before the drug can receive FDA approval.
In conclusion, although rare, PH patients may suffer severe kidney failure at any given time if the disease is not appropriately managed. Currently, treatment options may slow down disease progression, but without targeting the origin of the disease in the liver for excessive production of oxalate, the disease cannot be cured, and severe kidney failure can occur. The siRNA drug lumasiran has become the only FDA-approved medication with the potential to cure PH1 disease, and nedosiran has the potential to be developed as a siRNA drug to treat all types of PH diseases.
2. Targeting the Liver with Antisense Oligonucleotide and Small Interfering RNA Drugs for the Treatment of Familial Hypercholesterolemia
a. Epidemiology of familial hypercholesterolemia
People with FH are unable to sufficiently metabolize LDL cholesterol (LDL-C). They are thus more likely to develop ASCVD early in life if preventative lifestyle and pharmacologic measures are not taken. Up to 400 people in 100,000 are affected by FH, which is the most common monogenic disorder associated with ASCVD (Lui et al., 2021). Females and males are equally affected by this condition as it is caused by genetic differences that can be passed down at an equal rate in 50% by both sexes. It is more common in African ethnicities and less common in Asian groups. Latino and White populations fall in the middle (Toft-Nielsen et al., 2022). There is no significant difference in prevalence between children and adults. It should be noted that most studies done on FH occur in the Western high-income countries, so data are lacking on a global scale (Hu et al., 2020b).
b. Symptoms of familial hypercholesterolemia
FH is usually diagnosed by blood tests. Clinical findings indicative of FH include elevated total cholesterol and LDL-C and premature coronary heart disease. In homozygous FH, premature coronary heart disease can occur before the age of 20. On a physical exam, patients with FH may have arcus corneae, xanthelasma, tendon xanthomas, or tuberous xanthomas (Pejic, 2014).
c. Genetic causes of familial hypercholesterolemia
FH is a genetic disorder that is autosomal dominant. Carriers of the disease may inherit one or two alleles, making them heterozygous or homozygous, respectively. Homozygous FH is much rarer than heterozygous, manifesting in an approximately 1 in 100,000 to 1,000,000 individuals (Nohara et al., 2021), and is also the more extreme of the two (Cuchel et al., 2014). The most common mutations affecting the LDL-R gene are found in 80%–85% of cases. It is possible that apolipoprotein B (APOB) and proprotein convertase subtilisin/kexin type 9 (PCSK9) genes may be affected in rarer cases, accounting 5%–10% and 2% of cases, respectively (Pejic, 2014). The least common mutations are in the LDL-R adaptor protein 1 (LDLRAP1) gene. In other cases, clinically diagnosed FH patients do not have mutations in these genes. These patients are considered mutation negative, and the disease state may be due to polygenic causes instead (Benito-Vicente et al., 2018). The affected genes directly impact FH presentation. If LDLR is the mutated gene, plasma LDL-C levels are higher than if APOB or PCSK9 are affected. The most severe presentation is found if LDLR is affected (Lui et al., 2021).
d. Current treatment options for familial hypercholesterolemia
Lifestyle changes are the first recommendation for FH patients who must lower their LDL-C, but this by itself is not usually sufficient. For example, patients with FH are recommended to exercise regularly and refrain from smoking. Patients with comorbid conditions should be treated with standard therapies for those comorbidities. Examples of common comorbidities that can add risk for FH are hypertension and diabetes mellitus (McGowan et al., 2019). These lifestyle changes should be continued throughout pharmacological treatment of the best quality of life.
The first choice of most prescribed medications for FH is statins. Statins work by inhibiting b-hydroxy b-methylglutaryl-CoA reductase, preventing the step in the pathway leading to the production of cholesterol in the liver. Preventing cholesterol production in the body reduces LDL-C concentrations in the blood. The most common ADRs of statins are muscle pain and weakness (Benito-Vicente et al., 2018). The ADRs are more common among the high-intensity statins, atorvastatin 4–80 mg and rosuvastatin 20–40 mg. However, the high-intensity statins are the most efficacious for lowering LDL-C up to 50%–60%. Thus, they are recommended above other statins if tolerable to the patient (McGowan et al., 2019).
Niacin, bile acid sequestrants, and ezetimibe are also prescribed for the treatment of FH, often added onto statin therapy when not sufficient as monotherapy (Sjouke et al., 2011). Niacin impairs cholesterol and triglyceride synthesis. It may cause vasodilation and increased hepatic enzymes. Bile acid sequestrants complex with micelles made of bile acid and cholesterol, causing them to be excreted instead of absorbed. This reduces overall cholesterol levels and thus LDL-C. Bile acid sequestrants, such as colesevelam, may be effective, but they can also decrease the intestinal absorption of fat-soluble vitamins, which is a concern with their use. Ezetimibe blocks cholesterol uptake by inhibiting Niemann-pick C1-like 1 protein. This reduces cholesterol absorption through the intestine, lowering overall cholesterol content and LDL-C (Benito-Vicente et al., 2018).
A newer type of parenteral treatment of FH is human monoclonal antibodies. Alirocumab and evolocumab target PCSK9 protein, which normally breaks down LDL-R. Decreasing PCSK9 increases LDL-R so that more LDL-C can be taken up and brought to the liver for digestion. These have been used as monotherapy or in combination with other treatments and are well tolerated (McGowan et al., 2019). However, some patients end up with fatigue, muscle pain/soreness, and swelling near the injection site. There is an uncommon, but possible, risk of kidney and liver problems with these medications (Jhaveri et al., 2017).
e. Treatment of familial hypercholesterolemia with an antisense oligonucleotide drug mipomersen and a small interfering RNA drug inclisiran
i. Mipomersen
Mipomersen (brand name Kynamro) was developed by Ionis Pharmaceuticals and licensed by Genzyme Corporation as an adjunct therapy for lowing LDL-C, APOB, total cholesterol, and non–high-density lipoprotein cholesterol in patients with homozygous FH. Mipomersen received an approval by the FDA in January 2013 (Hair et al., 2013). It is added onto other lipid-lowering drug treatments, like statins and bile acid sequestrants (Rader and Kastelein, 2014). Mipomersen is a second-generation ASO drug with a 20-nucleotide-long gapmer (10 DNA nucleotides in the center flanked by 5 RNA nucleotides in both ends). All RNA nucleotides at both ends are generated with 2’-O-MOE chemical modifications (Crooke and Geary, 2013). The sequence and chemical modifications of mipomersen are shown in Fig. 3. Mipomersen binds to exon 20 and 21 of APOB mRNA (Fig. 4) and inhibits production of APOB-100 protein through RNase H–mediated degradation of APOB mRNA, which is needed to make plaque-forming lipids, like LDL (Hair et al., 2013). Mipomersen is administered as a subcutaneous injection of 200 mg weekly (Crooke and Geary, 2013).
As shown in Table 1, the PK, safety, and tolerability of mipomersen were determined by several phase 1 studies with healthy subjects (NCT01414881, NCT0133366, and NCT01061814). These studies demonstrated acceptable overall safety profiles with no indication of a systemic inflammatory response (Flaim et al., 2014). Based on this safety profile in healthy subjects, the long-term safety and efficacy of mipomersen were defined in either extended or new phase 2 studies to treat HF patients with or without high-risk statin intolerance, including an open-label extension study with 21 US patients (NCT00477594), a randomized, double blind, placebo-controlled study with 34 Dutch participants (NCT00707746), and an open-label dose-escalation study with 12 participants from the United States and Netherlands (NCT00280995). The results from these phase 2 studies concluded that mipomersen is effective as an adjunctive drug for lowering LDL-C (Patel and Hegele, 2010; Visser et al., 2012). However, elevated levels of alanine transaminase (ALT), aspartate transaminase (AST), and intrahepatic triglyceride content were recorded in some participants, which required long-term follow-up studies on liver safety.
The long-term safety and efficacy of mipomersen were further investigated in several phase 3 studies with much larger sample sizes, including an open-label extension study in 144 patients with FH or severe hypercholesterolemia (NCT0069109) (Santos et al., 2015a; Duell et al., 2016); a randomized, double blind, placebo-controlled study with 158 HF patients with high-risk hypercholesterolemia (NCT00770146) (Thomas et al., 2013; Santos et al., 2015a); the RADICHOL 1 study, with 51 homozygous FH patients (NCT00607373) (Raal et al., 2010; Santos et al., 2015a; Duell et al., 2016), and the FOCUS FH study, with 309 patients with FH and inadequately controlled LDL-C (NCT01475825) (Tragante et al., 2016). The double blind, placebo-controlled clinical trial (NCT00770146) found that mipomersen decreased LDL-C by 36.9%, APOB by 38%, and Lp(a) by 24%, all significantly better in comparison with placebo (Thomas et al., 2013). The study also found that injection site reactions and influenza-like symptoms were the most common ADRs of mipomersen. There were also abnormally increased ALT levels and hepatic fat levels in a portion of the patients treated with mipomersen. Long-term mipomersen treatment is also associated with a reduction in cardiovascular events in patients with FH, supported by other phase 3 trials (Duell et al., 2016).
As a result, the FDA granted approval for mipomersen in January 2013 (Hair et al., 2013). However, due to the elevated levels of ALT in a proportion of patients, the FDA also placed a Black Box Warning for the risk of hepatotoxicity on its label. It is also recommended that levels of ALT and AST are measured before using mipomersen and monitored throughout the course of treatment to assess changes in liver functions. After several years of use in the market with observed risk of hepatotoxicity, mipomersen was terminated by the FDA in 2019 (Lui et al., 2021).
Other ASO therapeutic agents are under development for the treatment of dyslipidemia, including vupanorsen and volanoesorsen. Vupanorsen is a second-generation, GalNAc-conjugated, chemically modified ASO designed by Ionis and Pfizer to target angiopoietin-like 3 mRNA expressed by the liver, which has shown to be able to reduce lipids and apolipoproteins in patients with dyslipidemia (Bergmark et al., 2022; Ahn et al., 2023). Volanoesorsen is a highly effective ASO agent designed to reduce elevated triglycerides in familial chylomicronemia syndrome (Lazarte and Hegele, 2021). Splice correction ASOs are also under investigation for the treatment of FH patients with mutations in LDL-R gene (McIntosh et al., 2021).
ii. Inclisiran
Inclisiran (brand LEQVIO) was developed by Alnylam and marketed by Novartis, which received approval by the FDA in December 2021 for the treatment of heterozygous FH and ASCVD for adult patients to lower LDL-C (Lamb, 2021). Inclisiran is a double-stranded siRNA drug (sequence and chemical modifications are shown in Fig. 3) that silences translation of the mRNA of PCSK9 to protein by binding to the 3′-UTR of the PCSK9 mRNA (Fig. 4) (Migliorati et al., 2022a). When the body produces less PCSK9 protein, more LDL-R removes more LDL-C from circulation by the liver. Inclisiran is conjugated to three GalNAc residues to improve liver targeting. Inclisiran is commonly used as an addition onto statins or the other similar drug options. However, if a patient is unable to take a statin, inclisiran can be used by itself. Its common ADRs are localized to the injection site as it is given as a subcutaneous injection. These include injection site reaction, pain, erythema, and rash (Stein et al., 2012). The preferred treatment plan includes doses of 284 mg at months 0, 3, and 6 and then every 6 months (Migliorati et al., 2022a).
Inclisiran mostly distributes to the liver due to its GalNAc conjugation. However, it is not known to be a substrate of liver cytochrome P450 enzymes or drug transporters. Thus, drug-drug interactions for this medication are minimal. It is instead degraded by nucleases. Inclisiran does not require hepatic or renal dose adjustments, but this medication has not been studied in patients with severe hepatic impairment or end-stage renal diseases (Stein et al., 2012; Ray et al., 2020).
As shown in Table 1, the safety and tolerability of inclisiran were evaluated in several phase 1 trials. A randomized, single blind, placebo-controlled phase 1 study (NCT02314442) with 70 participants with elevated LDL-C levels showed no serious ADRs and significantly reduced levels of PCSK9 and LDL-C (Fitzgerald et al., 2017). The safety and efficacy of inclisiran were established in extended phase 2 studies, including ORION-1 (NCT02597127) (Ray et al., 2018; Wright et al., 2020), ORION-2 (NCT02963311), and ORION-3 (NCT03060577) (Warden and Duell, 2021; Ray et al., 2023).
The major phase 3 trials that led to the FDA’s approval were ORION-8 (NCT03814187), ORION-9 (NCT03397121) (Raal et al., 2020), ORION-10 (NCT03399370) (Ray et al., 2020), and ORION-11 (NCT03400800) (Ray et al., 2020; Koenig et al., 2022). ORION-9 was a double blind, randomized, placebo-controlled trial utilizing only patients with heterozygous FH (Raal et al., 2020). The patient population was close to even between male and female, but there was still an overrepresentation of White patients. Across the time of the trial that lasted 18 months, the average decrease in LDL-C was 40%, and the decrease in PCSK9 was 61%. The most common ADRs were injection site reactions (Raal et al., 2020). Both ORION-10 and ORION-11 were randomized, double blind, placebo-controlled, parallel-group trials with sample sizes from 1500 to 3275. The patients involved in these trials had ASCVD or had a risk equivalent of ASCVD (including FH). The trial populations were highly male and White, which are not reflective of the affected population of FH alone. Inclisiran consistently reduced LDL-C by about 50% and PCSK9 by over 60% in these trials (Ray et al., 2020). There was a significant risk reduction in fatal and nonfatal myocardial infarctions (0.5) and strokes (0.2) in patients receiving inclisiran when compared with placebo. Death from cardiovascular causes was not reduced as much (0.9) (Ray et al., 2020).
After approval, inclisiran has been in the market for over 1 and half years. A real-world study had analyzed the early effects of inclisiran in a tertiary center lipid and cardiovascular risk clinic (Padam et al., 2022). The study concluded that inclisiran significantly lowered LDL-C at 2 months with efficacy similar to that reported in previous clinical trials with good tolerability. Further studies are needed for postmarket research on its efficacy on FH and toxicity, especially in highly affected minority groups and women. Several ongoing phase 3 trials are recruiting participants to evaluate inclisiran efficacy in various comparisons, including a comparison with inclisiran plus usual care to usual care alone in patients with a recent acute coronary syndrome (NCT04873934) and a study of inclisiran implementation to use care in patients with ASCVD and elevated DLD-C receiving maximally tolerated statin (NCT04929249).
In conclusion, nucleic acid therapeutics of ASO and siRNA drugs, particularly the FDA-approved inclisiran directed against PCSK9, have shown promising clinical implication in the management of FH disease in combination with maximally tolerated statin therapy.
Numerous agents using ASO and siRNA mechanisms are under development to target the liver to treat dyslipidemia-associated diseases in other systems (Gareri et al., 2022), including olezarsen (Karwatowska-Prokopczuk et al., 2022), an ASO agent developed by Ionis Pharmaceuticals targeting on APOC3 to lower triglycerides in blood, and ARO-APOC3 and ARO-ANG3, two siRNA agents developed by Arrowhead Pharmaceuticals targeting on APOC3 and ANGPIL3 for reducing triglycerides, LDL-C, apolipoprotein B, and angiopoietin-like protein 3 in patients with FH, familial chylomicronemia syndrome, severe hypertriglyceridemia, and mixed dyslipidemia (Fu et al., 2022; Gareri et al., 2022).
3. Targeting the Liver with a Small Interfering RNA Drug for the Treatment of Acute Hepatic Porphyria
a. Epidemiology of acute hepatic porphyria
AHP is a group of four genetic disorders caused by a defect in an enzyme involved in the heme biosynthesis pathway. The four different types of AHP are acute intermittent porphyria (AIP), hereditary coproporphyrin (HCP), variegate porphyria (VP), and δ-aminolaevulinic acid dehydratase deficiency porphyria (ADP) (Balwani et al., 2017). These defects cause repeat acute neurovisceral attacks that severely affect the patient’s mental and physical health. Precipitating factors induce the heme synthesis pathway through the rate-limiting enzyme, δ-aminolaevulinic acid synthase 1 (ALAS1), which produces an accumulation of the heme precursors aminolaevulinic acid (ALA) and porphobilinogen (PBG) in the liver, which then circulate through the rest of the body (Gouya et al., 2020). These intermediates are toxic at high levels, causing injury to the nervous system and a wide array of clinical manifestations.
AHP is considered a rare group of diseases and has a global prevalence of 5 per 100,000 individuals (de Souza et al., 2021). In the United States, less than 200,000 people are affected by AHP. The most common type is AIP, accounting for 80% of the total symptomatic cases of AHP (Balwani et al., 2020). Approximately 1 in 1600 Caucasians have mutations in the gene that causes AHP, but the penetrance among carriers is 1% in the general population and up to 50% in families with a history of the disorder (Balwani et al., 2020). AIP affects women significantly more than men, attacks most commonly begin postpuberty, and it recurs until menopause (de Souza et al., 2021). Founder effects in Scandinavia, southeast of Spain, and South Africa result in greater prevalence in these regions (Bonkovsky et al., 2019; de Souza et al., 2021). ADP is the rarest form of AHP and has only eight documented cases worldwide, with all of them being males (Mohan and Madan, 2022).
b. Symptoms of acute hepatic porphyria
All four AHP types present with acute attacks of pain due to central, peripheral, and autonomic nervous system dysfunction. The most common symptoms are abdominal pain, nausea and vomiting, constipation, hypertension, and tachycardia. Those who suffer from AHP often experience peripheral neuropathy like muscle pain and weakness, especially in the back, arms, and legs. Anxiety, delirium, hallucinations, insomnia, and seizures are neurologic symptoms that are also common among all forms of AHP (Bonkovsky et al., 2014; Ramanujam and Anderson, 2015). Patients are found to have reddish brown urine due to the oxidation of porphobilinogen to uroporphyrin (Bissell and Wang, 2015). Most heterozygotes of AIP remain asymptomatic or only experience one or a few attacks, but 5% of patients have four or more recurrent attacks per year. Those who suffer from AIP also have an increased risk for hepatocellular carcinoma, chronic hypertension, and end-stage renal disease (Ramanujam and Anderson, 2015). ADP presents similar severity to AIP, with the same neuropsychiatric and neuropathic symptoms with abdominal pain (Mohan and Madan, 2022). HCP and VP are less prevalent and less severe than AIP, although the symptoms during attacks are indistinguishable from AIP, other than the cutaneous manifestations like blistering skin lesions, bullae, erosions, ulcers, and milia. VP and HCP exhibit skin manifestations due to the buildup of photosensitizing porphyrins in the skin and blood vessels (Ramanujam and Anderson, 2015; Kothadia et al., 2022). There are several factors that can trigger these symptoms, including weight loss, surgery, anesthesia, environmental and lifestyle stressors like smoking and alcohol, diabetes, porphyrinogen drugs, and emotional stress (Bissell and Wang, 2015). AHP is an inherited group of diseases, but many individuals who have causing mutations do not experience clinical manifestations unless affected by these different risk factors. Symptoms can be difficult to attribute to AHP because they are nonspecific, which causes delays in diagnosis (Bonkovsky et al., 2014).
c. Genetic causes of acute hepatic porphyria
AHP is caused by genetic mutations in the genes of heme synthesis. Missense, nonsense, or other mutations in these genes in combination with environmental, nutritional, and hormonal factors lead to a serious decrease in heme and upregulation in ALAS1. AIP, HCP, and VP are autosomal dominant disorders. AIP is caused by a defect of the hydroxymethylbilane synthase (HMBS) gene, which leads to a deficiency in the enzyme porphobilinogen deaminase (Chen et al., 2016). HCP corresponds to a variant in the coproporphyrinogen-III oxidase (CPOX) gene that causes a defect in the enzyme coproporphyrinogen oxidase (Hasanoglu et al., 2011). VP is caused by a deficiency in the enzyme protoporphyrinogen oxidase due to a variant in the protoporphyrinogen oxidase (PPOX) gene (Whatley et al., 1999). Heterozygous carriers with defects in HMBS, CPOX, or PPOX genes have reduced enzyme activities to 50%, which does not cause a substantial deficiency of heme unless stimulated by precipitating factors. In ADP, enzyme activities are even lower due to both alleles being affected (Ramanujam and Anderson, 2015). Homozygous or compound heterozygous deficiencies of the affected enzymes in AIP, HCP, and VP are usually lethal in utero; very few children survive into adulthood due to extreme problems with the nervous system. ADP results from an abnormal activity of ALA dehydrase (ALAD) due to abnormal expression coded by the ALAD gene. ADP is an autosomal recessive disorder, which can cause either a heterozygous or homozygous deficiency of ALAD (Jaffe and Stith, 2007). Heterozygotes of the disorder are clinically asymptomatic (Bonkovsky et al., 2019).
d. Current treatment options for acute hepatic porphyria
Treatment of AHP primarily consists of identifying and avoiding the risk factors that induce attacks. Without exposure to precipitating factors, the heme biosynthesis pathway may not be activated, and attacks can be prevented. A patient would be screened for possible medications, for instance hormonal contraceptives, or underlying conditions like infection that could pose as precipitating factors. Smoking and alcohol should be avoided, and a patient’s dietary history should be reviewed (Anderson, 2019). If avoiding these triggers is not enough for patients who experience mild cases of attacks, then glucose overload therapy is an option. Glucose is administered as tablets or concentrated dextrose solutions in the initial stages of an attack (Balwani et al., 2017). For those who do not respond to glucose overload or experience more severe cases of attacks, hemin-based therapy (Panhematin or Normosang) is the next available option. ALAS1 is downregulated by the hemin groups that are taken by hepatocytes, which are oxidized iron protoporphyrin IX molecules. This results in the inhibition of ALA and PBG production, which inhibits clinical manifestations. Hemin is given daily for 4 to 5 days during an attack, and scheduled prophylactic infusions are most often given weekly or biweekly for prevention depending on the patients. This is because the effect of heme on ALAS1 only lasts 1 week, so repeated treatment is required for lasting effects (de Souza et al., 2021). Due to the hemin containing a significant amount of iron by weight, frequent infusions can lead iron overload to cause hepatic damage and fibrosis (Balwani et al., 2017). Hemin is often found to cause acute ADRs like headaches, fevers, and phlebitis, as well as chronic ADRs such as venous thrombosis, venous obliteration, and central venous catheter complications (Ventura et al., 2022). In some cases, women experience cyclic attacks due to hormone fluctuations from menstrual cycles, which can be managed with gonadotropin-releasing hormone or luteinizing-releasing hormone and is initiated in first few days of the cycle (de Souza et al., 2021). Liver transplantation has been found successful in extreme cases as the last resort for patients who experience severe debilitating attacks that do not respond to hemin therapy (de Souza et al., 2021).
e. Treatment of acute hepatic porphyria with the small interfering RNA drug givosiran
Givosiran, sold under the brand name Givlaari, was developed by Alnylam Pharmaceuticals to treat adults with AHP, which was approved by the FDA in November 2019 (Scott, 2020). Treatment with givosiran helps to reduce attacks, pain, and hemin use to improve the lives of patients with AHP. Givosiran is a double-stranded siRNA (sequence and chemical modifications on each nucleotide are shown in Fig. 3) targeting exon 5 of the ALAS1 mRNA (Fig. 4) to reduce production of ALAS1 protein in the liver (Majeed et al., 2022). Givosiran is covalently linked to a ligand containing three GalNAc residues that help directly to bind to ASGPR found on the membrane surface of hepatocytes, resulting in high specific delivery to the liver. After entering the hepatocyte, givosiran silences the ALAS1 mRNA through the RISC-mediated RNAi mechanism and reduces heme biosynthesis (de Souza et al., 2021). Givosiran is chemically modified with the addition of a 2’-deoxy-2’-F or a 2’-Ome group in the ribose sugar moieties, and PS linkages at the 5′ end of the siRNA strands to help prevent nuclease digestion of the siRNA stands (Majeed et al., 2022). It is administered once monthly by subcutaneous injection in a dosage of 2.5 mg/kg.
As shown in Table 1, the PK, safety, and tolerability of givosiran were first established in a phase 1 study (NCT02452372) with 40 participants with AIP. The study found that common ADRs included tolerable nasopharyngitis, abdominal pain, and diarrhea, and serious ADRs occurred in 15% of patients (Sardh et al., 2019). The long-term safety and efficacy of givosiran were further determined in a phase 1/2 study with AIP patients (NCT02949830). Givosiran resulted in reduced ALAS1 mRNA levels, nearly normalized levels of the neurotoxic intermediates, and a lower attack rate.
The phase 3 trial leading to the FDA’s approval is ENVISION (NCT03338816), a study to evaluate the long-term efficacy and safety of givosiran using 94 patients with AHP in a 6-month, double blind, randomized, placebo-controlled period (Balwani et al., 2020; Wang et al., 2022). Eligible patients had to be older than 12, have a confirmed genetic diagnosis of AHP and elevated levels of urinary ALA or PBG, and had at least two attacks within 6 months prior to the start of trial that required hospitalization or treatment with hemin. Half of the patients were given monthly givosiran (2.5 mg/kg) and the other half placebo for 6 months. Of the 94 patients with AHP, the annualized rate of composite porphyria attacks was 74% lower than the placebo group as well as reduced fasting levels of PBG and ALA. Additionally, greater than 50% of the patients did not receive hemin infusions. Givosiran-treated patients experienced reduced pain and less analgesic use, as well as improvement in physical health, mental health, and general quality of life. ADRs were experienced by both the givosiran and placebo groups. There were elevations in serum aminotransferase levels, 15% in the givosiran group and 2% in the placebo, which resolved by itself in most patients with continuous treatment. Only one patient was permanently discontinued from the trial due to persistently elevated ALT values. Additionally, there were renal adverse events such as increases in serum creatinine levels and decreases in estimated glomerular filtration rate, 15% in the givosiran group and 7% placebo, but were overall considered temporary. Approximately 25% of givosiran-treated patients faced mild injection site reactions (Balwani et al., 2020).
Before the approval of givosiran, hemin was the only FDA-approved therapy for AIP attacks in the United States (Blaylock et al., 2020). Givosiran has shown potential to significantly reduce the annualized rate of porphyria attacks as well as improved several other outcomes, including hemin use and pain (Syed, 2021). With only 3 years on the market, no real-world study on the usage of givosiran has been published yet. By directly targeting ALAS1 and heme biosynthesis, givosiran may become the first therapeutic agent for curing AIP disease. However, although generally well tolerated acceptable safety profile was defined in the clinical trials, givosiran may still increase the risk of hepatic and kidney toxicity, which needs to be monitored carefully during treatment.
4. Targeting the Liver with Antisense Oligonucleotide and Small Interfering RNA Drugs for the Treatment of Hereditary Transthyretin Amyloidosis
a. Epidemiology of hereditary transthyretin amyloidosis
hATTR is one of the most frequently researched SDLO due to its debilitating consequences and extrahepatic manifestations. Multiple aspects can be factored into the epidemiology of hATTR, such as location, ethnicity, age, and gender. The prevalence of hATTR is relatively low, with only approximately 50,000 patients (5 to 6 per 100,000) diagnosed worldwide (Hawkins et al., 2015). Of this number, approximately 10,000 patients have polyneuropathy, and about 40,000 patients have cardiomyopathy. In endemic areas like Portugal, Sweden, and Japan, incidence as high as 1 in 1000 has been reported (Hawkins et al., 2015). In Portugal, the location where hATTR was first diagnosed and researched, the prevalence is higher, with 1 in every 538 individuals having the condition. France and Brazil have also been named endemic areas in some studies due to moderate incidence, but they are not as populated with hATTR patients compared with Portugal, Sweden, and Japan. In the United States, hATTR has been diagnosed in about 6400 individuals, and those of European descent are at a greater risk of being affected (Schmidt et al., 2018). As healthcare technology, disease awareness, and genetic counseling advance, it is expected that the global number of reported cases will increase (Luigetti et al., 2020). Disease onset has been found to occur earlier in males compared with females, but the overall incidence is equivalent in both genders. All races and ethnicities are prone to hATTR, but there may be a higher frequency among Irish and African American patients. Although age of disease onset could vary based on each individual and the type of mutations that they have, a previous study has shown that hATTR is most evident in middle- to older-aged adults (Luigetti et al., 2020).
b. Symptoms of hereditary transthyretin amyloidosis
Signs and symptoms of hATTR can really vary from one individual to the next in terms of being mild or severe. Initial symptoms of hATTR include shortness of breath, numbness, swelling in the legs or ankles, bloody diarrhea, constipation, inflamed rippled tongue, easy bruising, and fatigue. As hATTR progresses, these symptoms can worsen and cause complications that require immediate medical attention. Peripheral polyneuropathy is one of the major systemic manifestations that includes both somatic and autonomic impairment. Common symptoms of autonomic neuropathy include orthostatic hypotension, sexual dysfunction, diaphoresis, and urinary tract infections. Somatic neuropathy is associated with voluntary movement dysfunction that progresses from a distal to proximal direction (Luigetti et al., 2020). In fact, hATTR with polyneuropathy has been categorized into different stages based on symptoms and need for assistance, with stage 0 being the least severe and stage 3 being the most severe (Hawkins et al., 2015). Patients can also present with serious cardiac, ocular, central nervous system, gastrointestinal, and renal manifestations. Examples include glaucoma, amyloid deposition on the iris or lens, conduction blocks, arrythmias, headaches, seizures, proteinuria, renal failure, unintentional weight loss, and carpal tunnel syndrome (Luigetti et al., 2020). Like polyneuropathy, cardiomyopathy is a common manifestation of hATTR, which can be accompanied by ventricular wall thickening, arrythmias, abnormal diastolic blood pressure, and heart failure (Hawkins et al., 2015).
c. Genetic causes of hereditary transthyretin amyloidosis
hATTR has a genetic basis and is characterized by the dissociation and misfolding of TTR protein, leading to abnormal deposition of amyloid fibril aggregates throughout the body. TTR protein, in its wild-type form, is responsible for transporting thyroxine and vitamin A along with retinol binding protein through circulation. TTR is a tetramer with each subunit containing 127 amino acids. Once dissociated, monomers contain eight antiparallel β-pleated sheets. This condition is typically classified as per two major systemic manifestations, polyneuropathy or cardiomyopathy, also known as familial amyloidotic polyneuropathy or cardiomyopathy, respectively (Ruberg and Berk, 2012). The major site of TTR protein synthesis is the liver, whereas smaller amounts come from the choroid plexus and retina.
The symptoms and patterns of hATTR distribution can be attributed to the genetic mutations in the TTR gene on chromosome 18, which contains four exons and three introns. hATTR has long been known to have a genetic cause; as research progresses and additional patient populations are discovered, new mutations continue to be identified. Currently, there are about 120 variants of the TTR gene that contribute to the disease (Luigetti et al., 2020). The most common mutation, Val30Met, is the point mutation at position 30, which results in the incorrect substitution of methionine instead of valine. This variant accounts for a significant percentage of the hATTR phenotypes around the world, most notably polyneuropathy in the endemic area of Portugal. Other mutations in the TTR gene can result in a mixed phenotype of polyneuropathy and cardiomyopathy or cardiomyopathy alone (Luigetti et al., 2020). The Val122Ile mutation results in mainly cardiomyopathy (Hawkins et al., 2015). The highest frequency of this mutation is seen in the African American population, whereas a greater percentage of European descendants are affected by the Val30Met mutation. These point mutations are inherited in an autosomal dominant pattern, which means both homozygous and heterozygous genotypes can cause hATTR. Since the TTR gene is located on an autosomal chromosome, there is no significant difference in the inheritance between male and female. However, the gender of the parents from whom the mutation is inherited can influence disease onset. A male child inheriting the mutation from his mother is at risk of early onset, whereas a female child inheriting the mutation from her father has conferred protection against the disease. Due to the autosomal dominant pattern of the disease, family members of any affected individual have a high risk of being carriers of the variant and should receive adequate genetic counseling as soon as possible (Luigetti et al., 2020).
d. Current treatment options for hereditary transthyretin amyloidosis
Current treatment options for hATTR are limited to symptomatic management, liver transplantation, and TTR-stabilizing drugs. Due to the multisystem involvement of this disease, management often involves a multifaceted approach. Patients are first started on symptomatic treatment of control of common mild systemic manifestations (Luigetti et al., 2020). These treatments can be a mixture of both nonpharmacologic and pharmacologic interventions like 9-α fludrocortisone, pacemaker implantation, or dialysis (Plante-Bordeneuve, 2014). More serious manifestations like polyneuropathy and cardiomyopathy require specific treatment plans with either orthotopic liver transplantation or TTR tetramer–stabilizing drugs.
For many patients, liver transplantation continues to be the first-line treatment. As per a retrospective analysis conducted by the Familial Amyloidotic Polyneuropathy World Transplant Registry, the 20-year survival rate after liver transplantation is 55.3% (Ericzon et al., 2015). Transplantation replaces the TTR gene mutations and allows wild-type TTR protein to be synthesized (Luigetti et al., 2020). This therapy is effective, with up to a 98% reduction in the mutant TTR protein (Hawkins et al., 2015). There are, however, some complications and disadvantages. Liver transplantation only prevents additional production of mutant TTR. So, preformed amyloid fibrils in the other organs could still induce newly synthesized wild-type TTR protein to dissociate and form aggregates as mutant TTR protein would. This phenomenon is referred to as “seeding” (Luigetti et al., 2020). There are also several adverse events associated with transplantation, such as hepatic artery thrombosis, increased risk of infection, and post-transplant lymphoproliferative disorder (Hawkins et al., 2015). Although there are benefits of liver transplantation, pharmacologic agents are needed as safer alternatives that are less invasives than tissue transplantation.
There are two main TTR stabilizers used in practice: tafamidis (brand name Vyndamax or Vyndaqel) and diflunisal (brand name Dolobid). Both work by mechanisms that stabilize the TTR tetramer. Like liver transplantation, the main issue with the TTR stabilizers is that they cannot prevent “seeding.” Nevertheless, TTR stabilizers are still used as the benefits outweigh the risks (Luigetti et al., 2020). Tafamidis is the more widely used TTR stabilizer and was approved by the FDA in 2019 for hATTR with cardiomyopathy. It can also be used off label in polyneuropathy. In the ATTR-ACT clinical trial, tafamidis was successful in reducing all-cause mortality and hospitalizations from cardiovascular complications and minimizing adverse events (Damy et al., 2021). Through this study, tafamidis got its approval and was considered a beneficial treatment option for cardiomyopathy (Hawkins et al., 2015). Diflunisal is also a TTR-stabilizing medication option for patients with hATTR, which belongs to the nonsteroidal anti-inflammatory drug class. Although not approved by the FDA for use in hATTR patients, it is used off label for polyneuropathy and cardiomyopathy. Diflunisal has been found to significantly reduce neuropathy progression and improve patient quality of life (Berk et al., 2013). Long-term use has been associated with characteristic nonsteroidal anti-inflammatory drug side effects like gastrointestinal bleeding, renal impairment, and cardiovascular stress (Luigetti et al., 2020).
Although the current treatment options for hATTR have had positive outcomes in patients, they do not target the genetic mutations specifically. Even after stabilizing the TTR tetramer, there is still overproduction of the mutant TTR protein. Both tafamidis and diflunisal are effective upon initiation, but they may not be able to sustain their effects for long-term use. For this reason, nucleic acid therapeutics have been developed. Nucleic acid therapeutics target the genomic basis of diseases and have longer-lasting effects regardless of the patient’s mutation type or disease stage at the initiation of therapy (Luigetti et al., 2020). For hATTR, four nucleic acid therapeutic agents have been developed, including two ASOs and two siRNAs. The goal of using these drugs in hATTR is to reduce the synthesis of the TTR protein by depleting the supply of the TTR mRNA (Hawkins et al., 2015).
e. Treatment of hereditary transthyretin amyloidosis with the antisense oligonucleotide drug inotersen; a potential ASO drug, eplontersen; and two small interfering RNA drugs, patisiran and vutrisiran
i. Inotersen
Inotersen (brand name Tegsedi) is an ASO drug developed by Akcea Therapeutics Inc. in affiliation with Ionis Pharmaceuticals Inc. This drug was approved by the FDA on October 5, 2018, for use in adults affected by hATTR with polyneuropathy (Joubran and Nguyen, 2022). Its mechanism of action is through binding to the 3′-UTR of the TTR mRNA (Fig. 4) for silencing its translation to TTR protein via RNase H–mediated degradation. Inotersen is not selective, so it degrades both mutant and wild-type TTR mRNA (Mathew and Wang, 2019). As a result, inotersen may lead to TTR protein deficits, thus requiring vitamin A supplementation (Joubran and Nguyen, 2022). Inotersen is a gapmer with 10 DNA nucleotides in the center flanked with five RNA nucleotides at both ends, which are chemically modified with 2’-MOE moieties (Fig. 3). The PS modifications are throughout the entire sequence. This allows higher ASO binding affinity to TTR mRNA, reduced immune stimulation, and less nuclease degradation (Dhuri et al., 2020). It is administered once weekly by subcutaneous injection in a dosage of 284 mg.
As shown in Table 1, the PK, PD, safety, and tolerance of inotersen have been determined in a series clinical trials. NEURO-TTR, a phase 2/3 randomized, double blind, placebo-controlled study (NCT01737398) with healthy subjects and patients with hATTR concluded that inotersen reduced polyneuropathy effectively and improved health-related quality of life in patients with hATTR (Benson et al., 2018; Yarlas et al., 2021; Karam et al., 2022). Significant ADRs, like thrombocytopenia, glomerulonephritis, and liver function impairment, were also reported in the clinical study (Benson et al., 2018, 2019). When inotersen was approved by the FDA, thrombocytopenia and glomerulonephritis were outlined in a Black Box Warning, and a Risk Evaluation and Mitigation Strategy (REMS) program was implemented. Prescribers must be certified within the REMS program by enrolling and completing training. The major contraindications for use are platelet counts, hypersensitivity, or history of glomerulonephritis because inotersen could potentially aggravate these conditions. Some of the less severe ADRs associated with inotersen are fatigue, injection site reactions, nausea, headaches, or fever, for which over-the-counter treatment options are permitted. As seen through clinical trials, inotersen has significant potential for bettering the lives of hATTR patients with polyneuropathy, but its toxicity needs to be monitored closely (Benson et al., 2018).
ii. Eplontersen
Eplontersen is also an ASO agent developed by Ionis Pharmaceutics Inc. in conjunction with AstraZeneca, but it is still in its investigational stage, awaiting for an approval by the FDA. As of March 7, 2023, the FDA accepted to review a new drug application for eplontersen to be used for hATTR adult patients with polyneuropathy. Eplontersen shares the same nucleotide sequence as inotersen, but it is conjugated to a triantennary GalNAc. The GalNAc3 moiety supports the ASGPR-mediated uptake of eplontersen into hepatocytes (Coelho et al., 2021). In other words, eplontersen is able to have more efficient uptake into hepatocytes over Kupffer cells or endothelial cells, unlike inotersen, which is less selective in cell-type uptake (Diep et al., 2022). GalNAc3 conjugation can also increase the potency of eplontersen about 20 to 30 times more than inotersen. Eplontersen is chemically modified to have fewer PS linkages in the backbone to improve the safety profile (Coelho et al., 2021). Eplontersen is administered once monthly by subcutaneous injection in a dosage of 120 mg.
As shown in Table 1, a good overall safety profile with infrequent treatment-emergent ADRs was found in a randomized, controlled, phase 1/2 study (NCT03728634), which concluded that eplontersen had improved safety and tolerability (Viney et al., 2021; Diep et al., 2022). Soon after these results became known, a phase 3 study, NEURO-TTRansform (NCT04136184), for eplontersen was initiated for stage 1 or 2 polyneuropathy. Eplontersen was compared against the active reference arm of inotersen. Eplontersen had positive improvements in all clinical endpoints and was also considered more convenient for patients due to its infrequent dosing regimen (Coelho et al., 2021). As the FDA reviews the new drug application for eplontersen in the coming months, Ionis Pharmaceuticals Inc. has already begun a phase 3 clinical trial, CARDIO-TTRansform (NCT04136171), to investigate eplontersen’s ability in reducing cardiovascular mortality and recurrence of significant cardiovascular events. If promising results are seen through the CARDIO-TTRansform study, there may be a hope for hATTR patients with cardiomyopathy to use eplontersen.
iii. Patisiran
Patisiran (brand name Onpattro) is an siRNA drug developed by Alnylam Pharmaceuticals Inc. and was approved by the FDA on August 10, 2018, for the treatment of adult hATTR amyloidosis (Hoy, 2018). Actually, patisiran is a first-of-its kind siRNA drug approved for the treatment of human diseases. Patisiran exerts its gene-silencing effects by binding to the 3′-UTR of the TTR mRNA (Fig. 4) by the RISC-mediated RNAi mechanism. Like inotersen, patisiran is not specific for mutant TTR mRNA, so both wild-type and mutant mRNA are depleted, and vitamin A supplementation is needed. While developing patisiran, its stability in circulation after administration was a major concern because the phosphodiester bonds in the backbone are subject to enzymatic degradation by endo and exonucleases (Urits et al., 2020). To counteract this, patisiran has been encapsulated in an LNP for optimum circulation time and efficient delivery into hepatocytes (Zhang et al., 2020a). To further enhance the stability of patisiran, 11 2’-OMe-modified sugar residues and four DNA residues have been added to the backbone (sequence and chemical modifications are shown in Fig. 3) (Urits et al., 2020). It is administered one dose every 3 weeks by IV infusion in a dosage of 0.3 mg/kg for body weight <100 kg or 30 mg for body weight >100 kg.
As shown in Table 1, in two phase 1 clinical studies, NCT01559077 and NCT01559077, patisiran reduced new TTR deposition in a dose-dependent manner (Coelho et al., 2013). This was followed by a dose-escalation phase 2 study (NCT01617967) to further illustrate the effectiveness of patisiran in reducing serum TTR levels with a generally well tolerated safety profile (Suhr et al., 2015). Another phase 2 study (NCT01961921) examined long-term treatment with patisiran and concluded that patisiran had an acceptable safety profile and was associated with improvement of polyneuropathy progression in patients with hATTR (Coelho et al., 2020). To further assess safety and efficacy, a phase 3 APOLLO (NCT01960348) clinical trial was conducted (Adams et al., 2017; Solomon et al., 2019). There was high subject retention, and all clinical outcomes favored patisiran over the placebo (Adams et al., 2018). Based on the results of these clinical trials, patisiran gained its FDA approval. Unlike inotersen, patisiran is administered infrequently every 3 weeks (Yu et al., 2020). Since patisiran must be diluted and administered intravenously, it can only be given by healthcare professionals. One of the most unique aspects about patisiran is the required premedication regimen with intravenous corticosteroids (dexamethasone 10 mg), oral acetaminophen 500 mg, intravenous H1 blocker (diphenhydramine 50 mg), and intravenous H2 blocker (ranitidine 50 mg) at least 60 minutes before the infusion begins. This is useful in minimizing infusion-related side effects. As one of the first siRNA therapeutics developed for hATTR, patisiran continues to be a safe and effective drug in the treatment of hATTR. Patisiran is also approved to be effective at reducing polyneuropathy for hATTR patients after liver transplantation (Schmidt et al., 2022). A real-world clinical study with Belgium hATTR patients showed a similar therapeutic response for altering the expected disease progression in most patients (De Bleecker et al., 2023).
iv. Vutrisiran
Vutrisiran (branded as Amvuttra) is the second siRNA drug that was approved by the FDA on June 13, 2022, for hATTR polyneuropathy in adults (Keam, 2022). Like patisiran, vutrisiran has also been developed by Alnylam Pharmaceuticals Inc. It is conjugated to GalNAc, similar to eplontersen, which increases potency, stability, and uptake into hepatocytes. The addition of this moiety is also advantageous because it eliminates the need for premedication (Adams et al., 2023). Vutrisiran has 2’-OMe, 2’-F, and PS modifications (Fig. 3) dispersed throughout the chemical structure to increase specificity and potency (Gangopadhyay and Gore, 2022). Like inotersen and patisiran, vutrisiran also binds to the 3′-UTR region of the TTR mRNA (Fig. 4). Notably, both inotersen and vutrisiran share a common targeting site excepting the presence of an additional nucleotide at the 3′-terminus of vutrisiran. It is administered one dose every 3 months by subcutaneous injection in a dosage of 25 mg.
HELIOS-A was the main phase 3 clinical trial (NCT03759379) to evaluate the efficacy and safety of vutrisiran in patients with hATTR polyneuropathy. This study showed good subject retention and statistically significant improvements in the primary and secondary endpoints (Adams et al., 2023). The all-cause mortality was relatively low in the vutrisiran treatment group compared with the patisiran active comparator group. Based on the result of this trial, vutrisiran gained its FDA approval. There are no major contraindications or warnings, besides the low vitamin A levels, for which supplementation is needed. The most common ADRs of this medication are arthralgia, dyspnea, and minor injection site reactions. In addition to the favorable safety profile, vutrisiran has been considered a preferable choice for patients because of the infrequent dosing regimen.
Even though vutrisiran has only been approved for the treatment of polyneuropathy, there is still hope for its approval in cardiomyopathy as well due to the ongoing HELIOS-B phase 3 clinical trial (NCT04153149). This trial is investigating vutrisiran’s efficacy in reducing all-cause mortality, recurrent cardiovascular events, and improving patient-reported outcomes. The study is estimated to be completed in February 2024. There is a possibility that hATTR cardiomyopathy patients will finally have a treatment option with nucleic acid therapeutics.
Even with all these developments in hATTR treatment options, there remain some knowledge gaps and areas of potential research. Many of the pharmacologic treatments currently available are only for patients with stage 1 or 2 polyneuropathy. So, treatments for advanced polyneuropathy and cardiomyopathy could be further explored. In patients with the Val30Met mutation and late-onset disease, there have been reports of high endogenous antibody concentrations from an immune response to misfolded TTR protein. If these antibodies could be used in patients with severe hATTR, it could potentially help target the amyloid deposits. Combination therapy is also another area of scientific exploration. Researchers have already tested tafamidis and diflunisal with patisiran and have reported good tolerability in patients (Lin et al., 2020). If more therapies like this could be combined, it could cover several mechanisms of action and have longer lasting effects (Hawkins et al., 2015).
5. Targeting the Liver with a Small Interfering RNA Drug for the Treatment of Hemophilia
a. Epidemiology of hemophilia
Hemophilia is an inherited bleeding disorder primarily in men, with a prevalence rate between 12 and 15 cases in 100,000 individuals in the United States in recent years (Soucie et al., 2020). There are two types of hemophilia: hemophilia A and B. Approximately 80% of hemophilia cases are hemophilia A. Both types of hemophilia affect people from all racial and ethnic groups, with a slightly higher rate in European Americans (15.7/100,000) than African or Hispanic Americans (12.4/100,000) (Soucie et al., 2020). Roughly estimated hemophilia patients between 29,000 and 33,000 are living in the United States today, with the majority receiving comprehensive care in specialized clinical centers.
Although hemophilia occurs primarily in men, women may also have hemophilia. Almost 20% of patients with mild hemophilia are female, who also need care at specialized hemophilia treatment centers in the United States (Miller et al., 2021).
b. Symptoms of hemophilia
Hemophilia is a condition in which blood does not clot properly. Hemophilia can cause spontaneous bleeding and bleeding following medical procedures and physically endured traumas (Sahu et al., 2011). Internal bleeding inside the body is more of a concern with this condition than bleeding from small cuts, which are not as worrisome unless someone has an extreme case of hemophilia where they may also bleed easily for no apparent reason. A general symptom of hemophilia is excessive bleeding from cuts and medical procedures. A person with hemophilia may experience nose bleeds more frequently and has a hard time controlling the bleeding and getting it to stop. Hemophilia patients may develop hematomas from bleeding into the skin or muscle. Commonly seen is pain, swelling, and tightness in joints, specifically in the knees and elbows, due to bleeding in the joints. Bleeding that is hard to control may be present during dental, mouth, and gums procedures (Shastry et al., 2014). In babies, there may be bleeding in the head following a difficult delivery, unnatural irritability without any apparent cause, and uncontrolled bleeding after circumcision in male babies (Andersson et al., 2019). Hemophilia patients may also experience unusual bleeding following vaccinations, such as COVID-19 vaccination (Al Hennawi et al., 2022).
In severe cases of hemophilia, a bump on the head can cause bleeding in the brain (Zanon and Pasca, 2019). This is a rare but serious complication of the disease that can be characterized by the presence of seizures, vomiting, sleepiness, weakness, painful unrelenting headache, double vision, and weakness.
Diagnosing hemophilia typically occurs in early childhood, and the median age at diagnosis is 36 months for mild, 8 months for moderate, and 1 month for those with severe hemophilia (Kulkarni et al., 2009). More than half of people diagnosed with hemophilia A have the severe form. Seventy-five percent of infants who were diagnosed in the first month of life are related to a family history or whose mothers were known carriers (Kulkarni et al., 2009).
c. Genetic causes of hemophilia
Hemophilia is caused by an X-linked chromosomal mutation that causes a bleeding disorder due to the loss of the coagulation F-VIII in hemophilia A and coagulation F-IX in hemophilia B (Santagostino and Fasulo, 2013). Hemophilia is inherited as an X-linked recessive condition. Severity of the disease is classified by the level of activity of coagulation factors in the blood (Santagostino and Fasulo, 2013). The lower the amount of coagulation factors present, the more severe the bleeding and the more severe disease a person has (Pavlova and Oldenburg, 2013). Typically, people have clotting factors forming a clot to stop bleeding. Still, in patients with hemophilia, the presence of clotting factors may be missing or present in very low levels. Hemophilia can be inherited and is known as congenital hemophilia, characterized by the presence of low levels of a clotting factor caused by genetic mutations (Wang et al., 2017). People can also develop hemophilia with no family history of the condition, known as acquired hemophilia, where the person’s immune system attacks coagulation F-VIII or F-IX in the blood. Acquired hemophilia can be associated with multiple sclerosis, pregnancy, cancer, or drug reactions. Since hemophilia is an X-linked condition, it is found primarily in males as opposed to females since females have two X chromosomes and males have one X and one Y chromosome. For females to have hemophilia, they would need both X chromosomes from the mother and father for the disease to present itself. Males receive the X chromosome that contains the defective gene with hemophilia from the mother. Most females with the faulty gene for hemophilia on one X chromosome show no signs and symptoms of the condition but may have some unusual bleeding in some cases. A female who has one chromosome that contains the gene for the condition can pass it onto 50% of her children.
The molecular basis of hemophilia A and B is extremely diverse and caused by many different types of mutations, with single-nucleotide mutations, inversions, missense mutations, and deletions being found to cause both hemophilia A and B (Castaman and Matino, 2019). In hemophilia A, the shared genetic defect leading to this condition is intron 22 inversions, and in hemophilia B, a common congenital defect is missense mutations (Santagostino and Fasulo, 2013). Studies have shown that the type of mutation affecting either clotting F-VIII or F-IX is correlated with the plasma levels of these coagulation factors, meaning that more severe gene defects are associated with more severe cases of hemophilia (Castaman and Matino, 2019).
Many females who are carriers of the gene for hemophilia also have low factor expression, which can result in easy bruising, bleeding within joints, and heavy menstrual periods. The factor may be so low that some females who are carriers get diagnosed with hemophilia (Miller et al., 2021).
d. Current treatment options for hemophilia
There is no cure for both hemophilia A and B as it is a lifelong condition. However, current available treatments can improve quality of life by reducing the frequency and severity of bleeding episodes.
Current hemophilia treatments include replacement therapy that injects clotting factor(s) concentrates (Aledort et al., 2019). This includes a bypassing agent of either plasma-derived factor concentrates or recombinant factor concentrates. Plasma-derived concentrates come from human plasma filtered extensively to select the appropriate clotting factor needed for the patient. Recombinant factor concentrate comes from genetically engineered DNA technology. By injecting these concentrations of the clotting factors missing in the patient, it helps the blood clot properly for prophylactic and episodic care.
Due to its safety in preventing any blood-borne virus, a large portion of the hemophilia replacement therapy is the recombinant factor concentrates. Unlike plasma-derived concentrates, the recombinant F-VIII and F-IX do not contain any plasma or protein that could contribute to blood-borne viruses. Gene recombinant therapy offers the potential for a cure for patients with hemophilia by establishing continuous endogenous expression of F-VIII or F-IX following the transfer of a functional gene to replace the hemophilic patient's defective gene (Nathwani, 2019).
Treatment of hemophilia A would target those with congenital F-VIII deficiency. Instead of directly replacing the clotting factor, it can reduce the frequency of bleeding episodes. Hemlibra, also known as emicizumab, is a product prescribed for patients with hemophilia A (Lenting et al., 2017). Emicizumab is a macromolecule drug administered subcutaneously as it is an antibody consisting of two antigen-binding domains. Antibodies can recognize both the enzyme F-IXa and the substrate F-X. By simultaneously binding enzyme and substrate, emicizumab mimics some part of the function exerted by the original cofactor, F-VIII, in that it promotes colocalization of the enzyme-substrate complex (Lenting et al., 2017). This leads to more thrombin molecules being generated, which can aid in stopping excessive bleeding. Emicizumab demonstrated statistically and clinically significant improvements in several types of bleeding outcomes such as treated bleeds, all bleeds, treated joint bleeds, and treated spontaneous bleeds (Oldenburg et al., 2017).
Patients with hemophilia B would be recommended with the congenital F-IX. BeneFix, also known as Nonacog alfa, is the recombinant F-IX used for the treatment of hemophilia B (LAMBERT et al., 2007).
The recombinant treatment has been efficacious. However, there have been ADRs reported with use. ADRs are frequent after F-VIII and can occur also with infusions after the first dose. Hence, recent use of F-VIII outside of hospital settings, such as home treatment, should be implemented with warning and caution (Batorova et al., 2022).
e. Treatment of hemophilia with the potential small interfering RNA drug fitusiran
Fitusiran, also known as ALN-AT3SC, is a siRNA therapeutic agent under development by Alnylam Pharmaceuticals in collaboration with Sanofi. Fitusiran is designed to target antithrombin (AT) mRNA to lower the production of AT protein in the liver and its levels in the blood, which can rebalance the imbalanced coagulation system caused by deficiencies in other clotting proteins, such as F-VIII (hemophilia A) or F-IX (hemophilia B), and promote hemostasis in hemophilia (Sehgal et al., 2015). Fitusiran is a synthetic double-stranded siRNA using the Alnylam GalNAc conjugate platform for hepatocyte-specific delivery. Its physiologically based pharmacokinetics and PD feature for silencing AT protein in the liver was proven in preclinical species of mice and monkeys (Sehgal et al., 2015; Ayyar et al., 2021). It is administered once monthly by subcutaneous injection in a dosage of 80 mg.
In clinical studies (Table 1), the PK, PD, and safety of fitusiran were first assessed in a phase 1 trial (NCT02035605) by Sanofi, completed in July 2017. The study enrolled 51 participants with healthy volunteers and patients with moderate or severe hemophilia A or B who did not have inhibitory alloantibodies (Pasi et al., 2017). Subcutaneous administration of fitusiran resulted in dose-dependent lowering of the AT protein levels on blood and increased thrombin generation in participants with hemophilia A or B. Fitusiran showed a desirable safety profile, with mild injection site reactions being the most common adverse events (Pasi et al., 2017). A further open-label phase 1 study using 17 male participants with moderate or severe hemophilia A or B with alloantibody inhibitors also concluded that fitusiran was generally well tolerated, could lower AT levels from baseline, and resulted in improved thrombin generation (Pasi et al., 2021).
Sanofi further conducted an open-label extended phase 1/2 study (NCT02554773) using 37 participants with hemophilia A or B with or without inhibitors to examine the longer-term durability, safety, and efficacy of fitusiran. The study concluded that fitusiran resulted in a lower annualized bleeding rate over a median of 2.6 years in patients with hemophilia A or B (Pipe et al., 2020).
Two multicenter, open-label, randomized phase 3 clinical trials have been completed by Sanofi. In the study of ATLAS-INH (NCT03417102), 60 patients with hemophilia A or B with inhibitors were recruited for assessing the efficacy and safety of fitusiran prophylaxis. The study concluded that subcutaneous fitusiran prophylaxis resulted in statistically significant reductions in annualized bleeding rates in participants with hemophilia A or B with inhibitors, with 66% of participants having zero bleeds in comparison with 5% in control (Young et al., 2023). The study ATLAS-A/B (NCT03417245), using 120 patients with hemophilia A or B without inhibitors, also concluded that fitusiran prophylaxis resulted in significant reductions in annualized bleeding rates compared with on-demand clotting factor concentrates and had no bleeding events in 51% of participants in comparison with 5% in control (Srivastava et al., 2023). The significant ADRs reported in these studies included abdominal pain, increased ALT and AST, cough, asthma, respiratory tract infection, nasopharyngitis, arthralgia, gastritis, and headache. Both phase 3 studies support that fitusiran has the potential to be transformative in the management of all people with hemophilia.
Two more phase 3 trials are nearly completed, but the results still need to be published. The ATLAS-PPX (NCT03549871) aims to determine the efficacy and safety of fitusiran prophylaxis in 80 severe hemophilia A or B patients previously receiving factors or bypassing agent prophylaxis. The study was completed in January 2022. Based on the press release by Sanofi, 63.1% of adults and adolescent patients treated with fitusiran experienced zero-treated bleeds in comparison with 16.9% of patients with prior factors or bypassing agent prophylaxis. The study met the primary endpoint and demonstrated that fitusiran prophylaxis significantly reduced bleeding episodes compared with primary factor or bypassing agent prophylaxis.
Another ongoing phase 3 study, ATLAS-PEDS (NCT03974113), aims to determine the efficacy and safety of fitusiran prophylaxis in male pediatric subjects aged 1 to less than 12 years with hemophilia. The study is estimated to be completed in August 2023. Sanofi's other ongoing phase 3 studies will determine the long-term safety and efficacy of fitusiran (ATLAS-OLE, NCT03754790) with a larger study cohort (355 participants) and test injection under the skin for preventing bleeding episodes (ATLAS-NEO, NCT05662319).
In conclusion, fitusiran has shown promising results in reducing bleeding episodes and improving quality of life in participants with hemophilia A and B. With further studies and trials, fitusiran would be approved by the FDA as a primary treatment option for hemophilia patients with better outcomes.
6. Targeting the Liver with Potential Antisense Oligonucleotide and Small Interfering RNA Drugs for the Treatment of Atherosclerotic Cardiovascular Disease
a. Epidemiology of atherosclerotic cardiovascular disease
ASCVD is prevalent and the leading cause of vascular diseases worldwide (Herrington et al., 2016). In the United States, the prevalence of ASCVD was 24 million, approximately 10% of the American adult (over the age of 21) population in 2019 (Gu et al., 2022). Lower socioeconomic non-White people have an elevated risk of ASCVD (Morris et al., 2018).
b. Symptoms of atherosclerotic cardiovascular disease
Atherosclerosis is a type of arteriosclerosis, which occurs when the arteries become thick and stiff, restricting blood flow from the heart to organs and tissues. Atherosclerosis is the buildup of plaque, which can include fats, cholesterol, and other substances in the arteries. This buildup narrows the arteries, preventing blood flow, and can lead to the development of several cardiovascular disorders such as coronary heart disease, peripheral arterial disease, and stroke.
c. Genetic causes of atherosclerotic cardiovascular disease
ASCVD is a complex disease with many contributing factors, including genetic variations. However, there is not one specific gene mutation that causes ASCVD. Rather, multiple genetic variants and environmental and lifestyle factors contribute to an individual's risk of developing ASCVD.
Many genes have been correlated with the development of ASCVD, including those involved in lipid metabolism, inflammation, and endothelial function (Nayor et al., 2021). These genes encode multiple proteins, including Apolipoprotein E (APOE), PCSK9, methylenetetrahydrofolate reductase (MTHFR), and tumor necrosis factor (TNF). Mutations in these genes have been associated with a higher risk of developing ASCVD. The APOE protein is involved in the transport of lipids, more specifically cholesterol (Mahley and Rall, 2000). The PCSK9 protein is involved in regulating LDL-C levels. Drugs typically target PCSK9 to lower LDL-C levels in high-risk patients (Lepor and Kereiakes, 2015). The MTHFR protein is involved in the metabolism of folate and homocysteine (Raghubeer and Matsha, 2021). The TNF protein is associated with the regulation of inflammation (Kalliolias and Ivashkiv, 2016). Variants of the genes encoding these proteins, alongside other risk factors such as diabetes and obesity, increase the risk of ASCVD (Nayor et al., 2021).
It is also important to note that these genetic factors alone are not solely responsible for the developmental risk of ASCVD. Environment and lifestyle factors also play a significant role in the development of ASCVD (Lechner et al., 2020).
In addition to genetic variations, elevated levels of Lp(a) have also been correlated with an increased risk for developing ASCVD (Reyes-Soffer et al., 2022). Lp(a) is a lipoprotein composed of an LDL-like particle attached to a glycoprotein. Lp(a) is often associated with the transport of cholesterol and other lipids, wound healing, and promotion of inflammation and plaque formation (Orsó and Schmitz, 2017). An elevated Lp(a) level is an independent signifier of ASCVD (Enas et al., 2019).
d. Current treatment options for atherosclerotic cardiovascular disease
Current treatment options for ASCVD focus on reducing the risk of developing cardiovascular events. These include many lifestyle changes, medications, and invasive procedures. These lifestyle changes include exercising regularly, cessation of smoking, weight management, and healthy eating, all of which are associated with a reduced risk of cardiovascular events. Current medications include statins, antiplatelet agents, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and β blockers (Heidenreich et al., 2022). These medications aim to reduce the risk of cardiovascular events through many different pathways: statins lower cholesterol levels in the blood, antiplatelet agents aim to prevent blood clots, ACE inhibitors and ARBs lower blood pressure and prevent heart failure, and β blockers aim to reduce blood pressure and heart rate. In severe cases of ASCVD, invasive procedures such as angioplasty or coronary artery bypass surgery can be implemented to improve blood flow to the heart and further reduce the risk of cardiovascular events.
e. Treatment of atherosclerotic cardiovascular disease with the potential antisense oligonucleotide drug pelacarsen and the potential small interfering RNA drug olpasiran
Although lifestyle modifications and pharmacological interventions such as statins can help reduce the risk of ASCVD, new therapies still need to target the underlying mechanisms of the disease. One potential approach is to use RNA-based therapeutics, including ASO and siRNA drugs, to selectively target and inhibit the expression of proteins involved in lipid metabolism and inflammation. Two RNA agents that have shown promise in clinical trials are an ASO agent, pelacarsen, and a siRNA agent, olpasiran, both targeting elevated levels of Lp(a).
Pelacarsen, also termed Ionis-APO(a)-LRx, AKCEA-APO(a)-LRx, or TQJ230, is an ASO therapeutic agent under development by Ionis and Novartis for the treatment of ASCVD by targeting elevated Lp(a) levels (Karwatowska-Prokopczuk et al., 2021). Pelacarsen is a chemically modified ASO to enhance its stability, improve its pharmacokinetic properties, and reduce the risk of immune activation. The chemical structure of pelacarsen includes a backbone made of nucleotides, which are linked together by phosphodiester bonds. However, pelacarsen also contains several chemical modifications, including PS linkages, 2'-MOE modifications, and a cholesterol conjugate at the 3′ end of the molecule (Warden and Duell, 2022). These modifications enhance the drug's stability, improve its tissue distribution, and enhance its cellular uptake. Pelacarsen is administered once monthly by subcutaneous injection in a dosage of 120 mg.
As shown in Table 1, the safety, tolerability, PK, and PD of pelacarsen were determined by Ionis and Novartis in several phase 1 studies, including NCT02900027 (56 healthy volunteer participants with elevated triglycerides), NCT05337878 (29 healthy Japanese participants), and NCT05026996 (17 participants with mild hepatic impairment). These studies showed an overall improvement in the lipid profile with a favorable safety and tolerability profile (Alexander et al., 2019). A phase 2 study (NCT03070782) with a randomized, double blind, placebo-controlled, dose-ranging trial involving 286 patients with established ASCVD and elevated Lp(a) levels was further conducted and completed in November 2018. The study also showed that pelacarsen reduced Lp(a) levels in a dose-dependent manner in patients with ASCVD disease (Tsimikas et al., 2020) and also had neutral to mild lowering of LDL-C levels (Yeang et al., 2022). A phase 3 study Lp(a) HORIZON (NCT04023552) is under intervention with an actual enrollment of 8323 participants to assess the impact of Lp(a) lowering with pelacarsen on major cardiovascular events in patients with ASCVD (Plakogiannis et al., 2021). The study is expected to be completed in May 2025. No approved pharmacological therapies are currently available to lower Lp(a) effectively. Lp(a) HORIZON study may provide a solution for 8 million patients with ASCVD disease.
Olpasiran, also known as AMG 890, is a siRNA therapeutic agent under development by Amgen to treat ASCVD by inhibiting translation of the Lp(a) protein in hepatocytes, leading to a marked reduction in Lp(a) levels in the circulation (O’Donoghue et al., 2022a). Olpasiran is also chemically modified to enhance its stability, improve its pharmacokinetic properties, and reduce the risk of immune activation. These modifications include PS linkages to strengthen stability, 2'-MOE modifications to enhance its affinity to the target sequence, and a GalNAc conjugation to increase its uptake in the liver. Olpasiran is administered once monthly by subcutaneous injection in a dosage of 75 mg.
The PK, PD, and tolerability of olpasiran were studied by Amgen in several populations, including NCT04987320 (24 Chinese participants with elevated serum Lp(a) levels), NCT05481411 (25 participants with various degrees of hepatic impairment), NCT05489614 (32 participants with normal and multiple degrees of renal impairment), and 27 healthy Japanese and non-Japanese participants (Sohn et al., 2022). These studies found that olpasiran was well tolerated with no clinically significant adverse events or laboratory abnormalities.
Supported by the phase 1 studies on safety and tolerability, a phase 2 clinical trial (NCT04270760) with a double blind, randomized, placebo-controlled study was conducted and completed in November 2022 by Amgen to evaluate the efficacy, safety, and tolerability of olpasiran in 290 participants with elevated Lp(a) levels. The study concluded that olpasiran therapy significantly reduced Lp(a) concentrations from 70% to 100% in a dose-dependent manner in patients with established ASCVD disease (O’Donoghue et al., 2022b). A multicenter phase 3 trial OCEAN(a) (NCT05581303) is under intervention with an estimated enrollment of 6000 participants to assess the impact of olpasiran on major cardiovascular events in patients with ASCVD disease and elevated Lp(a) levels. The study is estimated to be completed in December 2026. Olpasiran has shown to be a promising treatment approach that produces a profound and sustained reduction in Lp(a) concentrations in the circulation in clinical trials.
Both ASO and siRNA drugs under development have shown great promise and may provide a solution for millions of patients worldwide with ASCVD disease.
7. Targeting the Liver with Potential Small Interfering RNA Drugs for the Treatment of α-1 Antitrypsin Deficiency-Associated Liver Disease
a. Epidemiology of α-1 antitrypsin deficiency-associated liver disease
AATLD is not a rare disease but a disease rarely diagnosed, with approximately 180,000 individuals worldwide (de Serres, 2003). AATLD is extremely under-reported in the United States, with fewer than 10,000 individuals diagnosed, but an estimated prevalence may be over 100,000 cases (30 per 100,000) (Stoller and Brantly, 2013; Ashenhurst et al., 2022). A long delay may happen between initial symptoms and first detection, and the need to see multiple physicians may also be required before diagnosis (Stoller and Brantly, 2013).
b. Symptoms of α-1 antitrypsin deficiency-associated liver disease
AATLD affects many different areas of the body, the most prominent being the liver. However, the disease has also been known to affect the lungs as well (Teckman and Mangalat, 2015). AATLD is caused by the synthesis of an atypical and mutated α-1 antitrypsin protein (AAT). The misfolded protein forms polymers that prevents it from leaving the hepatocytes and accumulating in the liver (Silverman et al., 2013). This, in turn, leads to unwanted cellular consequences, such as endoplasmic reticulum stress, hepatocellular injury, inflammation, and apoptosis. Over time, these effects can result in liver diseases, ranging from mild asymptomatic enzyme elevations to hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (Teckman and Mangalat, 2015). Individuals may also develop lung diseases like emphysema or chronic obstructive pulmonary disease (Anzueto, 2015). Symptoms affecting the lungs include dyspnea, cough, and wheezing. Many individuals with AATLD develop serious conditions like asthma, emphysema, or chronic bronchitis (Tejwani and Stoller, 2021). Liver dysfunction is a major indicator of AATLD, especially in children (Lin et al., 2019). Over 50% of the children with AATLD have liver dysfunction. When under 18, individuals with AATLD face cirrhosis and other life-threatening disorders about 5% of the time (Nelson et al., 2012). Symptoms have also been displayed in neonatal children. A significant symptom seen in infants shortly after birth is cholestatic jaundice and hepatitis (Nelson et al., 2012).
c. Genetic causes of α-1 antitrypsin deficiency-associated liver disease
AATLD is caused by mutations in the serine protease inhibitor 1 (SERPINA1) gene encoding the AAT protein. More than 200 mutations have been identified in the SERPINA1 gene in various populations (Foil, 2021). Approximately 40% of these mutations are thought to cause some forms of AATLD (Seixas and Marques, 2021). Due to the sheer number of mutations that cause AATLD, it affects individuals very differently. Individuals with AATLD who experience liver diseases are commonly seen with a homozygous Z allele (Bouchecareilh, 2020). These individuals are noted as PiZZ homozygous. The PiZZ genotype is frequently referred to as the most severe form of AATLD as it results in severe clinical presentations (Santos and Turner, 2020). That being said, other genotypes, like PiSZ and PiMZ, can also present severe conditions (Santos and Turner, 2020).
d. Current treatment options for α-1 antitrypsin deficiency-associated liver disease
Currently, there are no pharmacological therapies that can cure AATLD. However, numerous options can be used to manage the disease and its corresponding effects. There are also promising pharmacological interventions being developed to cure AATLD. Despite these efforts, the primary course of action involves treating the symptoms of AATLD. As mentioned above, these symptoms can take many forms, which results in a high variability in the ways AATLD can be treated.
A major method of treating AATLD is to manage it. This is primarily done alongside a physician monitoring the effects and complications of chronic liver diseases, which may arise from having AATLD (Teckman, 2013). Prescribers treating the complications of chronic liver diseases include everything from bleeding to hepatocellular carcinoma. These symptoms are usually managed by physicians who meet regularly with the patient to ensure that there are no other complications related to AATLD (Teckman, 2013). By doing this, less severe cases can be managed, often resulting in healthy lives for the patients.
In severe cases, liver transplantation must be performed. This is usually only performed when cirrhosis or hepatocellular carcinoma is present (Carey et al., 2013). Liver transplantation is the only way to truly cure AATLD (Zamora and Ataya, 2021).
AAT augmentation therapy is a replacement therapy for treating patients with AATLD who have emphysema (Barjaktarevic and Miravitlles, 2021). This therapy uses normal AAT protein derived from the blood of healthy donors to increase the amount of AAT in the lungs of patients with AAT deficiency. AAT augmentation treatment has been shown to slow the progression of emphysema and lung destruction when caused by AATLD (Edgar et al., 2017). Despite this, AAT augmentation is simply a way to subdue the symptoms of AATLD in the lung. To be clear, AAT augmentation treatment does not subdue AATLD’s effects on the liver but only helps to treat AATLD’s effects in the lungs.
e. Treatment of α-1 antitrypsin deficiency-associated liver disease with the potential small interfering RNA drugs fazirsiran and belcesiran
siRNA therapeutic strategy has been applied to reduce the production of mutated AAT proteins in the livers of AATLD patients. Two siRNA drugs, fazirsiran and belcesiran, are in the development stages.
Fazirsiran, also known as ARO-AAT, is a siRNA therapeutic agent currently being developed by Arrowhead and Takeda to treat AATLD. Fazirsiran is an investigational siRNA agent containing a synthetic, double-stranded siRNA duplex conjugated to GalNAc to facilitate endosomal uptake and intracellular delivery in hepatocytes (Wooddell et al., 2020). Fazirsiran has been shown to reduce hepatic Z-AAT polymer, restore endoplasmic reticulum and mitochondrial health, normalize expression of disease-associated genes, reduce inflammation, and prevent tumor formation in the PiZ transgenic mouse model (Wooddell et al., 2020). Fazirsiran also provided robust and persistent knockdown of ATT levels in PiZZ patients (Turner et al., 2018). It is administered once monthly by subcutaneous injection in a dosage of 100 mg.
As shown in Table 1, the safety, tolerability, pharmacokinetics, and pharmacodynamics of fazirsiran were determined in a phase 1 study (AROAAT-1001, NCT03362242) with 45 participants, completed in October 2018, which provided a strong base of the safety profile to support two ongoing phase 2 studies, AROAAT-2002 (NCT03946449) and SEQUOIA (NCT03945292).
For AROAAT-2002 (NCT03946449), with a start date in December 2019 and an estimated completion in August 2024, the clinical trial had 16 participants split up into three cohorts. Every cohort received doses of fazirsiran subcutaneously on day 1 and week 4 followed by every 12 weeks dosing. However, the different cohorts had different endpoints. For example, cohort 1, consisting of four individuals, received a 200-mg dose of fazirsiran until week 24. Cohort 1b, consisting of four individuals, also had an endpoint of week 24 but only received 100-mg subcutaneous injections. Cohort 2, composed of eight individuals, received 200-mg subcutaneous injections and had an endpoint of week 48. The goal of this clinical trial was to measure the baseline change in mutant protein (Z-AAT) concentrations. The study results showed that all patients had reduced levels of Z-AAT protein in the liver at the end of the trial (Strnad et al., 2022). Another important finding of the study is that there were no adverse side effects in any individual.
For SEQUOIA (NCT03945292), with a start date in August 2019 and completion in November 2021, the clinical trial consisted of 40 individuals diagnosed with AATLD (Remih et al., 2021). Patients with baseline fibrosis received one of three doses (25, 100, or 200 mg) of fazirsiran subcutaneously. The goal of this study was also to determine critical aspects including safety, efficacy, and tolerability of fazirsiran. All three of these cohorts showed dramatic decreases in the mutant AAT protein, with the largest reduction being 94% with a dose of 200 mg (Remih et al., 2021). Another interesting point is that 50% of the individuals saw improvements in their lung fibrosis.
Since the findings of both phase 2 clinical trials saw effective reductions in the mutant Z-AAT protein, fazirsiran can play a significant role in treating AATLD. This statement is further compounded by the fact that neither trial saw individuals with significant adverse side effects. A randomized, double blind, placebo-controlled phase 3 study has been started since March 2023, with an estimated enrollment of 160 participants, to further evaluate the efficacy and safety of fazirsiran in the treatment of AATLD (NCT05677971). The study is estimated to be completed in March 2027. Fazirsiran has the potential to provide life-changing treatment to people suffering from AATLD.
Belcesiran, also known as DCR-A1AT, is another siRNA therapeutic agent under development by Dicerna Pharmaceuticals to treat AATLD. Belcesiran contains 22 bases of ribonucleotides on the guide strand (antisense) targeting the SERPINA1 mRNA and 36 bases on the passenger strand (sense) with tetraloop hairpin. The sequence is conjugated with 4 GalNAc molecules using GalXC RNAi technology invented by Dicerna scientists (Kumar and Turnbull, 2023). Multivalent GalNAc moieties are linked to siRNA at the region of the tetraloop hairpin (Hu et al., 2020a), which introduces a stable complementary structure in the siRNA sense strand. This design erases sense strand–mediated off-target effects and increases the stability and specificity of siRNAs targeting their mRNA targets. This allows GalXC RNA technology to have many benefits, from high specificity to hepatocytes and increased duration of action to a straightforward dosing process. Belcesiran is subcutaneously delivered while still providing specificity to liver cells, particularly hepatocytes.
After proving the concept and defining the pharmacokinetic features of belcesiran in preclinical studies, a phase 1 clinical trial was initiated in October 2019 and completed in July 2021 (NCT04174118) (Remih et al., 2021). A phase 2 clinical trial was undertaken in February 2021 and is estimated to be completed in September 2023 (NCT04764448).
For the phase 1 clinical trial, two treatment arms were used. One cohort received belcesiran subcutaneously. The other cohort received a placebo containing 0.9% NaCl saline, also subcutaneously. The goals of the phase 1 clinical trial were to identify the incidence of adverse effects, significant physical examination findings, and changes in 12-lead ECGs, all over the span of 2 months. Changes in AAT protein concentrations were also tested. These measurements were taken at varying time frames, from 3 days to up to 57 days. Unfortunately, due to its recent development, the final data of the phase 1 clinical trial have not been posted. However, interim data from phase 1 showed no serious adverse side effects with belcesiran.
For the phase 2 clinical trial, three cohorts were used, each with their own placebo and belcesiran treatment arms. Cohorts were differentiated based on the length of treatment with belcesiran or placebo. Cohort 1 received belcesiran/placebo injections for 24 weeks. Cohorts 2 and 3 received the same injections for 48 and 96 weeks, respectively. This trial aims to evaluate belcesiran’s effects on adult patients with PiZZ AATLD. Criteria like safety, tolerability, and pharmacokinetics are measured. This is being done through a variety of measurements taken through 12-lead ECGs, physical examinations, vital sign measurements, and clinical laboratory tests. The efficacy of belcesiran on the treatment of AATLD will be released after the clinical trial is completed.
As there is no cure for AATLD, belcesiran provides hope for patients suffering from chronic symptoms. Although its phase 1 and phase 2 clinical trials data are not published, the GalXC siRNA technology provides hope for patients struggling with AATLD.
AATLD is an extremely dangerous and often misunderstood disease. With its effects having a wide range of presentations, it is often underdiagnosed. Even if it is diagnosed, there is no pharmacological cure to treat AATLD. The only way to treat AATLD is to manage the side effects or undergo extreme interventions like liver transplantation. However, the future is bright for individuals suffering with AATLD as belcesiran and fazirsiran have shown promising results (Remih et al., 2021). Fazirsiran has already demonstrated effectiveness in its two phase 2 clinical trials. With a phase 3 clinical trial being initiated, fazirsiran shows promise. Despite belcesiran having little results, its GalXC technology shows tremendous upside in its ability to deliver the drug to hepatocytes effectively. siRNA therapeutics have clearly made dramatic jumps recently and provide promising hope to people suffering from chronic diseases like AATLD.
8. Targeting the Liver with a Potential Small Interfering RNA Drug for Treatment of Complement-Mediated Diseases
a. Epidemiology of the complement-mediated diseases
The complement system is the critical part of the innate immune system that defends our body from microbial infections and clears injured cells (Noris and Remuzzi, 2013). In normal conditions, the complement system is tightly controlled and regulated to avoid attack on autologous tissues. The complement system consists of nine central components (C1 to C9). When some components are hyperactivated, they may drive a severe inflammatory response in numerous organs and may result in various CMD. For example, hyperactivation of C5 is associated with the CMD of PNH (Kulasekararaj et al., 2022), aHUS (Tanaka et al., 2021), and IgAN (Rizk et al., 2019).
PNH is a rare chronic hematologic disease associated with inappropriate complement activity on blood cells, resulting in intravascular hemolysis, thromboembolic events, and organ damage (Kulasekararaj et al., 2022). The prevalence of PNH in the United States is estimated to be 1.2 to 1.3 cases per 100,000 people, which is similar across sex and increases with age (Jalbert et al., 2019).
aHUS is a rare genetic disease that causes tiny blood clots to form in blood vessels, blocking blood flow to essential organs and causing kidney failure, heart disease, and other serious health problems. The prevalence rate of aHUS is less than 1 per 100,000 individuals worldwide (Yan et al., 2020).
IgAN is the most common glomerulonephritis worldwide, with an incidence rate of approximately 2.5 per 100,000 in the last two decades (Rodrigues et al., 2017; Storrar et al., 2022), but has significant geographical variations, with a higher prevalence rate in East Asia, then in Europe and Africa (Magistroni et al., 2015).
b. Symptoms of the complement-mediated diseases
Symptoms of PNH include blood clots, weakness, fatigue, headache, abdominal pain, back pain, dark urine, easy bleeding, and shortness of breath. The symptoms of aHUS may include paleness, swelling, fatigue, nausea, vomiting, drowsiness, and hypertension. The most common symptoms of IgAN are visible blood in urine (hematuria), proteinuria, hypertension, flank pain, and ankle swelling (edema).
c. Genetic causes of the complement-mediated diseases
A somatic mutation in the phosphatidylinositol glycan anchor biosynthesis class A gene has been associated with deficiency of the glycosylphosphatidylinositol anchor, leading to complement-mediated hemolysis in PNH patients (Takeda et al., 1993). The genetic mutations in the complement factor H (CFH) gene have been considered as the most common cause of inherited aHUS (Wong and Kavanagh, 2018). Mutations in other complement factor genes, including C1, C3, and CHB, are also associated with aHUS. Due to the striking phenotypic variations, the genetic basis of IgAN is still unclear (Feehally and Barratt, 2015).
d. Treatment options of the complement-mediated diseases
To manage the symptoms and reduce potential damage to the kidneys, several classes of medications are normally the options for the treatment of CMD. Antihypertensive drugs, such as ACE inhibitors and ARBs, are normally prescribed to lower blood pressure to prevent or delay further kidney damage for CMD patients (Dillon, 2004). Lipid-lowering medicines are also used to control cholesterol levels and slow down kidney damage in CMD patients with high cholesterol levels (Moriyama et al., 2014). Diuretics can be applied to control swelling and edema (Uzu et al., 2005).
To target the hyperactivated complement component proteins, the FDA has approved monoclonal antibody and peptide drugs to treat CMD diseases. The liver is the major organ producing the majority of the complement proteins, and Kupffer cells in the liver are the important immune cells (Thorgersen et al., 2019). A pivotal part of liver homeostasis is to control the complement system in immune responses. Blocking the production of the key complement component proteins in the liver provides a strategy to treat CMD due to hyperactivation of the complement component proteins. Eculizumab and ravulizumab are humanized monoclonal antibodies against C5, approved by the FDA for the treatment of PNH and aHUS (Dmytrijuk et al., 2008; McKeage, 2019). The genetic variants in the C5 gene are associated with response to eculizumab (Nishimura et al., 2014). Pegcetacoplan, a PEGylated pentadecapeptide binding to C3 protein, is also approved by the FDA to treat PNH patients (Hoy, 2021).
Recently, a newly developed humanized monoclonal antibody named pozelimab has shown full inhibition function against C5 activity in mice (Latuszek et al., 2020) and nonhuman primates (Devalaraja-Narashimha et al., 2022). Developed by Regeneron Pharmaceuticals, pozelimab is under a series of clinical trials to evaluate its efficacy and safety in treating the CMD diseases.
In addition to using antibodies against C5, siRNA has also been considered as another approach to block C5 production in hepatocytes for treating CMD diseases.
e. Treatment of the complement-mediated diseases with the potential small interfering RNA drug cemdisiran
Cemdisiran, under development by Alnylam Pharmaceuticals, is a siRNA agent with chemically modified double-stranded RNA sequences, in which the sense strand contains 21 nucleotides, and the antisense strand contains 25 nucleotides (Badri et al., 2021). A tri-antennary GalNAc is conjugated to the 3′-end of the sense strand for hepatocyte-specific delivery. Through endocytosis into the hepatocytes, cemdisiran specifically targets C5 mRNA, resulting in decreased hepatic synthesis and lower circulating levels of C5 protein. Thus, cemdisiran is expected to ameliorate the signs and symptoms of CMD, offering a novel approach for treating the CMD diseases. Cemdisiran is administered once weekly by subcutaneous injection in a dosage of 200 mg.
The PK, PD, safety, and tolerability of cemdisiran were first assessed by a single- and multiple-ascending dose phase 1/2 study (NCT02352493) with 32 healthy adult volunteers and 30 PNH patients. A long PD duration of action in the liver for robust C5 suppression was found in the study, with acceptable safety profiles (Badri et al., 2021). The PD and safety results support further evaluation of cemdisiran in CMD as either monotherapy or in combination with a C5 inhibitor antibody. Two open-label phase 1 studies (NCT04601844 and NCT04940364) conducted by Regeneron Pharmaceuticals further evaluated the PK, PD, safety, and tolerability of cemdisiran in combination with pozelimab in healthy adult volunteers, supporting a potential long-acting treatment of PNH and other CMD diseases.
Alnylam has conducted a phase 2 study (NCT03841448) together with Regeneron to evaluate the efficacy of cemdisiran in adults with IgAN. The treatment with cemdisiran resulted in a 37% percent mean reduction of C5 protein from baseline in the urine relative to placebo, showing the expected efficacy with IgAN patients. The overall safety and tolerability profile of cemdisiran in the phase 2 study supports continued clinical development. Alnylam is also conducting a phase 2 study, DANCE (NCT03999840), in patients with aHUS to switch from eculizumab to cemdisiran.
Currently, Regeneron is performing a phase 2 study (NCT04888507) to examine cemdisiran and pozelimab combination therapy in adult patients with PNH who switch from eculizumab therapy and a phase 2 study (NCT04811716) with PNH who have received pozelimab monotherapy. These phase 2 studies are nearly completed. Although their results have not been published, cemdisiran may become another therapeutic option besides monoclonal antibodies to suppress C5 production in the liver to treat various CMD diseases.
Three phase 3 studies are currently in the stages of participant recruitment. The ACCESS-EXT (NCT05744921) study will examine the long-term safety, tolerability, and effectiveness of pozelimab and cemdisiran combination therapy in patients with PNH. The ACCESS-1 (NCT05133531) study will evaluate the efficacy and safety of pozelimab and cemdisiran combination therapy in patients with PNH who are complement inhibitor treatment-naïve or have not received complement inhibitor therapy. The NIMBLE (NCT05070858) study will determine the efficacy and safety of pozelimab and cemdisiran combination therapy in patients with symptomatic generalized myasthenia gravis.
In conclusion, by directly targeting C5 mRNA in hepatocyte cells, the siRNA drug cemdisiran, under development by Alnylam, has shown promising efficacy to robustly suppress production of C5 protein and elevated levels in the circulatory system with favorable safety profiles. The combination therapy of cemdisiran with the C5 monoclonal antibody pozelimab may provide a therapeutic solution for managing various CMD diseases, including PNH, aHUS, and IgAN.
9. Summary of Antisense Oligonucleotide and Small Interfering RNA Drugs for the Treatment of the Systemic Diseases of Liver Origin
In a summary, the discussed SDLO diseases in this article cover six rare diseases of CMD, PH, AHP, hATTR, hemophilia, and AATLD, and two common diseases of FH and ASCVD, due to the definition of a rare disease as a condition that affects fewer than 200,000 people, or 60 per 100,000 in the United State (Chung et al., 2021). The most discussed SDLO diseases have balanced prevalence rates between males and females, except for AHP, which has significantly higher prevalence rates in females (approximately 90%) than in males (Wang, 2021), whereas hemophilia is a predominant disease for males because it is an X-linked disorder (Bowen, 2002). Most SDLO diseases preferably occur with higher prevalence rates in adults than in children, except for PH (predominantly in childhood) and FH (similar in childhood and adults). Furthermore, most SDLO diseases have been found to be associated with genetic mutations in specific genes involved in key pathways for disease progression (Table 2). Some diseases are associated with genetic mutations of a single gene, such as AGXT for PH1, GRHPR for PH2, HOGA1 for PH3, and TTR for hATTR. However, most SDLO diseases are caused by genetic mutations in multiple genes and nongenetic factors.
Treatment options for the SDLO diseases
In addition to the liver, the discussed SDLO diseases mainly affect the kidneys (aHUs, IgAN, PH, and hATTR), heart and cardiovascular system (hATTR, FH, and ASCVD), blood (PNH and hemophilia), nervous system (AHP and hATTR), lung (AATLD), and gastrointestinal tract (hATTR) (Table 2). The drugs used to treat the SDLO diseases include drugs that manage the SDLO symptoms and further prevent damage of the affected organ systems. However, these medications cannot cure the diseases without targeting the liver, the origin of the diseases. Before developing antibodies and ASO/siRNA drugs, liver transplantation was commonly the only curative strategy for certain cases of SDLO diseases, such as PH, AHP, hATTR, and AATLD (Table 2). After a decade of effort in the discovery and development of nucleic acid therapeutics by the pharmaceutical industry, mainly led by Alnylam, Ionis, Dicerna, Arrowhead, Novartis, Amgen, and Takeda, currently, the FDA has approved seven ASO and siRNA drugs for the treatment of the SDLO diseases discussed here. These RNA-based therapeutic agents include lumasiran for PH1; givosiran for AHP; inotersen, patisiran, and vutrisiran for hATTR; and mipomersen (withdrawn) and inclisiran for FH. Other eight ASO and siRNA drugs have entered phase 3 clinical trials with desirable efficacy and safety profiles and great potential to be approved by the FDA in next few years, including cemdisiran for CMD, nedosiran for PH1/PH2/PH3, eplontersen for hATTR, fitusiran for hemophilia, fazirsiran and belcesiran for AATLD, and pelacarsen and olpasiran for ASCVD.
The ASO and siRNA drugs used for the treatment of the SDLO diseases are chemically synthesized in sodium salt and formulated in a sterile, preservative-free, clear, colorless-to-yellow solution with a typical injection volume of 1.5 mL (Table 3). Dosing with these drugs is typically in the range of 100 and 300 mg, with a few exceptions, either lower than 100 mg or higher than 300 mg. All drugs are mainly administrated by subcutaneous injection, except for patisiran, which is administrated by intravenous infusion. Most ASO and siRNA drugs can be administrated once per month, with some given once every 3 months (lumasiran and vutrisiran) and even up to once every 6 months (inclisiran). Only inotersen and mipomersen (weekly) as well as patisiran (every 3 weeks) need to be administrated more frequently (less than a month). The long duration and less frequent administration of the ASO and siRNA drugs make them to have significant advance over small chemical drugs that are frequently administered daily oral dosing, more than once per day (Thakur et al., 2022). A less frequent drug administration regimen, as in the case of ASO and siRNA drugs, should lead to better patient adherence and medication treatment outcomes (Kulkarni et al., 2021).
Formulation, dosing, and administration of the ASO and siRNA drugs for the treatment of the SDLO diseases
III. Challenges and Future Perspectives
ASOs and siRNAs hold great promise as powerful tools for gene regulation and therapy. They work by specifically targeting and degrading mRNAs to silence gene expression and provide therapeutic benefits for various diseases, including SDLO diseases. However, the clinical use of these molecules is still limited by potential toxicity challenges, such as severe thrombocytopenia, hepatotoxicity, and immune system activation.
A. Challenges on Adverse Drug Reactions and Toxicity of the Antisense Oligonucleotide and Small Interfering RNA Drugs
ASO and siRNA drugs are generally considered safe and tolerable drug classes. The major and minor ADRs and toxicity have been characterized for the ASO and siRNA drugs to treat the SDLO during the discovery and development in preclinical and clinical studies (Alhamadani et al., 2022; Wu et al., 2022; Ranjbar et al., 2023). Table 4 summarizes the information from the FDA’s drug labeling on the “Black Box Warning,” “Warnings and Precautions,” common ADRs (>5%), less common ADRs (<5%), and immunogenicity of the seven FDA-approved ASO and siRNA drugs, which can be grouped into no toxicity (lumasiran and inclisiran), mild toxicity (vutrisiran, patisiran, and givosiran), and severe toxicity (inotersen and mipomersen).
ADRs and toxicity of the FDA-approved ASO and siRNA drugs for the treatment of the SDLO diseases
Lumasiran and inclisiran have no “Black Box Warning” or “Warning and Precautions.” The most common ADRs are injection site reactions, which are mild and manageable. No evidence on drug-induced immunogenicity is found for this group of drugs.
Vutrisiran, patisiran, and givosiran have no “Black Box Warning.” Only mild toxicity is listed in the “Warnings and Precautions” section. Reduced serum vitamin A is the major listed warning for vutrisiran and patisiran, which can be corrected by supplementing vitamin A (Shah et al., 2021). Anaphylaxis, hepatic toxicity, renal toxicity, and injection site reactions have been listed in the “Warning and Precautions” for givosiran. The severity of the toxicity is in the manageable range. Some listed common ADRs for these drugs include arthralgia, dyspnea, muscle spasms, fatigue, and erythema, which should be carefully monitored. Drug-induced immunogenicity is not indicated or inconclusive due to limited available data.
A “Black Box Warning” for severe thrombocytopenia and glomerulonephritis is present in the drug labeling for inotersen. A Black Box Warning is the FDA’s most stringent warning of severe toxicity for drugs in the market (Delong and Preuss, 2023). Glomerulonephritis (3%) and thrombocytopenia (3%) were found in the inotersen-treated group, and one death was associated with a grade 4 thrombocytopenia in the NEURO-TTR phase 3 clinical trial (NCT01737398) (Benson et al., 2018). Because of the risks of severe thrombocytopenia and glomerulonephritis, the FDA has enforced the use of inotersen only through a restricted distribution program under a REMS program. In addition to thrombocytopenia and glomerulonephritis, other severe conditions, including stroke and cervicocephalic arterial dissection, inflammatory and immune effects, liver effects, hypersensitivity reactions, uninterpretable platelet counts, and reduced serum vitamin A, are listed in the “Warnings and Precautions” for inotersen. Many common and less common ADRs are found to be associated with inotersen treatment. Positive detection for inotersen-induced antibodies indicates immunogenicity response caused by the treatment.
A “Black Box Warning” for severe hepatotoxicity appears on the labeling of mipomersen, which can also only be used under a REMS program. Hepatotoxicity, the elevation of transaminases, hepatic steatosis, injection site reactions, and flu-like symptoms are listed in the “Warnings and Precautions” for mipomersen. Mipomersen is also positive for immunogenicity response.
Some ASO and siRNA candidate drugs for the SDLO diseases are even terminated in developmental stages due to unacceptable safety issues. For example, revusiran is a first-generation candidate siRNA drug developed by Alnylam to treat hATTR that has gone through nonclinical studies (Sutherland et al., 2020) and phase 1 (NCT01814839) and phase 2 (NCT02292186) clinical trials. However, the phase 3 study ENDEAVOR (NCT02319005) observed mortality imbalance associated with revusiran, and no clear causative mechanisms could be identified (Judge et al., 2020). Therefore, Alnylam decided to terminate the development of revusiran (Garber, 2016). Another such example is PRO-040201 (also known as TKM-ApoB), a siRNA candidate developed by Tekmira Pharmaceuticals to knock down the expression of APOB protein and lower cholesterol. A phase 1 study (NCT00927459) identified flu-like symptoms, indicating potential immune stimulation with dose escalation, which resulted in the termination of the clinical studies. Understanding the molecular mechanisms of toxicity is essential for optimizing design and development of better ASO and siRNA drugs.
B. Understanding of Antisense Oligonucleotide–Induced Thrombocytopenia
Following ASO treatments, drug-induced thrombocytopenia has been observed in preclinical animal studies and clinical trials. The most prevalent type of thrombocytopenia associated with ASOs is mild, temporary, and related to the dosage. Approximately 10% of ASOs have been reported to cause this type of thrombocytopenia (Crooke et al., 2016). Mice administered with a first-generation ASO frequently experienced a reduction in platelet count. PS modification can be another risk factor for thrombocytopenia due to its capability to bind to platelet proteins, such as platelet factor 4, and activate platelets to various extents (Sewing et al., 2017). However, after 2′-OMe modification was applied, the incidence was reduced. Clinical trial data in the Ionis integrated safety database confirmed that ASOs with 2’-MOE modifications did not show the generic effect on platelet numbers, but specific sequences may associate with clinically insignificant platelet declines (Crooke et al., 2017).
The second type of thrombocytopenia is evident and has recently been reported in clinical trials (Crooke et al., 2016). The ADRs were uncommon but severe in studies involving monkeys and only occurred after repeated exposure (Frazier, 2015). Some studies have reported that there was no apparent relationship between the dose and the response (Crooke et al., 2016), but it is not possible to rule out a connection between the dose and the effect, or a threshold effect, due to the small number of animals studied per dose. In monkeys, it manifested as multiple bleeding episodes and lethargy that necessitated stopping the medication. Although platelet counts may increase after discontinuing the drug, thrombocytopenia can recur if the drug is reintroduced. Additionally, there have been suggestions that the mechanisms of thrombocytopenia may differ between species, and it is currently unclear whether humans and monkeys share similar mechanisms.
Moreover, during the NEURO-TTR clinical trial for inotersen, 54% of patients who received inotersen had platelet counts below 140,000 cells/mm3, compared with only 13% of those who received the placebo. Three cases of glomerulonephritis were also reported in patients who received inotersen (Benson et al., 2018). As a result, the FDA Black Box Warning for thrombocytopenia and glomerulonephritis was included in the final product label for inotersen. Although there has been significant interest and research on the subject, the underlying mechanism(s) of ASO-induced thrombocytopenia still needs to be better understood. However, the distinct presentations and kinetics of the mild and severe types of the condition suggest that different mechanisms may be responsible for their pathogenesis.
C. Understanding of Antisense Oligonucleotide and Small Interfering RNA–Induced Immunogenicity Response
The FDA drug labeling for both inotersen and mipomersen indicates positive immunogenicity responses. Its underlying molecular mechanisms have yet to be elucidated. Toll-like receptors (TLRs) 3, 7, 8, and 9 are specialized receptors located in endosomes that recognize specific nucleic acids and trigger an innate immune response (Gay et al., 2014; Lind et al., 2022). Whereas TLR3 responds to double-stranded RNA, TLR7/8 can respond to single-stranded RNA (Meng and Lu, 2017), and TLR9 recognizes sequences containing unmethylated cytosine-guanine (CpG) motifs, which are potent ligands of TLR9 (Bauer et al., 2001). siRNA-based therapeutics can induce similar unwanted effects via TLRs because of their ability to activate these receptors. Recent structural studies have shown that PS-ASO-TLR9 activation is a complex process involving diverse nucleic acid structures that can activate TLR9 (Chan et al., 2015) and the existence of a second ligand docking site. TLR9 activation necessitates dimerization, with one TLR9 protomer recognizing the CpG nucleotides whereas the other protomer binds to the phosphate backbone (Ohto et al., 2015). When TLR9 is activated in the endosome, it recruits Myd88, which triggers a series of signaling pathways that results in the activation of nuclear factor κ-light-chain-enhancer (NF-κB). The activation of NF-κB leads to the production and expression of proinflammatory signals, which can contribute to immune responses and inflammation in the body. This pathway is initiated when TLR9 detects the presence of CpG nucleotides. The activation of TLR9 is a highly regulated process that involves the trafficking of the receptor from the endoplasmic reticulum to the plasma membrane surface and finally to endosomal structures. Once in the endosome, TLR9 dimerizes and recruits Myd88, which leads to the activation of NF-κB and the expression of proinflammatory signals (Gay et al., 2014). Non-CpG PS-ASOs can also trigger innate immune responses through the TLR9 pathway (Paz et al., 2017; Pirie et al., 2019), but no structural explanation exists for this phenomenon. There is also evidence that TLR9-independent mechanisms can come into play (Senn et al., 2005). It is important to note that the innate immune responses can be influenced by changes to the PS-ASO's length, sequence, backbone modification, and 2'-ribose modification (Drygin et al., 2005; Paz et al., 2017; Anderson et al., 2021). However, the precise mechanisms behind these effects remain unclear, and there is no established medicinal chemical approach to reducing innate immune activation. Furthermore, there is a lack of understanding regarding the trafficking of non-CpG innate immune PS-ASOs and their engagement with TLR9.
D. Strategies to Address the Challenges
Addressing these challenges of toxicity requires careful consideration of various strategies. One strategy for addressing toxicity challenges of siRNA and ASO therapeutics is chemical modifications of the molecules. These modifications can improve stability and reduce toxicity by preventing immune recognition and increasing cellular uptake. Chemical modifications to the sugar or phosphate backbone or adding protective groups to the nucleobases have improved pharmacokinetics, reduced toxicity, and increased efficacy. Another strategy for overcoming the toxicity challenges of siRNA and ASO therapeutics is the development of efficient delivery strategies. One of the biggest challenges with siRNA and ASOs is their efficient delivery to the target cells. Various delivery strategies have been developed, such as lipid nanoparticles, conjugation to cell-penetrating peptides, and electroporation. The choice of delivery strategy depends on the target tissues, cell types, and specific therapeutic application. Target selection is another crucial strategy to address the challenges of toxicity. The specificity of siRNA and ASOs can be improved by careful target selection. Choosing targets that are highly expressed in the disease tissues or cells and have minimal off-target effects can reduce toxicity and improve efficacy. Immunomodulation is also a potential strategy for reducing toxicity. siRNA and ASOs can trigger an immune response that can lead to toxicity. Immunomodulatory strategies, such as coadministration with immunosuppressive agents, can help reduce the immune response and improve safety. A recent holistic analysis on the intrinsic and delivery-mediated toxicity of siRNA therapeutics provides a comprehensive review on understanding of mechanistic toxicity of siRNA drugs in clinical studies (Ranjbar et al., 2023).
Another challenge is to produce biologic nucleic acid agents quantitively with high quality for research and development purposes. The recently developed recombinant technologies may facilitate the purposes for biomedical research and RNAi-based therapeutics (Cronin and Yu, 2023; Traber and Yu, 2023). A bioengineered siRNA has shown to be effective to interfere with key pathways in cancer cells to improve chemosensitivity (Li et al., 2018). This bioengineering platform uses a novel transfer RNA fused pre-siRNA carrier-based technology to offer consistent and high-yield production of biologic RNA molecules from Escherichia coli fermentation. Due to the production and process of RNA molecules in living cells, the platform can better recapitulate the properties of natural RNAs as superior research tools for the studies of absorption, distribution, metabolism, and excretion and toxicity of ASO and siRNA drugs.
In conclusion, although siRNA and ASOs hold great promise as powerful tools for gene regulation and therapy, their clinical use is limited by potential toxicity challenges. Addressing these challenges requires careful consideration of various strategies, including chemical modifications, efficient delivery strategies, target selection, dose optimization, immunomodulation, and preclinical testing. By utilizing these strategies, researchers can improve the safety and efficacy of siRNA and ASO therapeutics and bring them closer to clinical application.
IV. Conclusion and Final Remarks
This article has summarized the following information: 1) SDLO; 2) epidemiology, symptoms, genetic basis, and treatment options of eight SDLO diseases, including three diseases caused by abnormal metabolism and homeostasis of small compounds (oxalate, cholesterol, and heme) in the liver and five diseases caused by excessive production of wild-type or mutated proteins in the liver [TTR, F-VIII, F-IX, Lp(a), and C5]; and 3) discovery and development of the seven FDA-approved ASO and siRNA drugs and the eight ASO and siRNA agents currently in late clinical trials (phase 3) targeting mRNAs encoding the critical proteins in the progress of the SDLO diseases.
The distinct pharmacological and toxicological profiles of the ASO and siRNA drugs are summarized in Table 5. SiRNA drugs offer highly specific gene silencing through RNA interference, leading to potent target inhibition and the potential for sustained effects. However, they often require intricate delivery systems and can induce off-target effects, raising concerns about unintended gene silencing. On the other hand, ASO drugs offer a more straightforward delivery route and exhibit reduced off-target effects. They act through steric hindrance or RNase H–mediated cleavage, allowing modulation of gene expression. Nonetheless, ASO drugs may exhibit reduced potency and efficacy compared with siRNA, necessitating higher doses for therapeutic effect. Furthermore, both siRNA and ASO drugs can trigger immune responses, which could contribute to potential toxicities. Careful consideration of these factors is crucial when developing therapeutic strategies for SDLO.
Emphasizing the aspects of comparisons between siRNA and ASO drugs
Challenges of major ADRs and toxicity are analyzed and strategies to address the challenges are discussed. The information in this article is expected to stimulate more studies to improve the safety and efficacy of siRNA and ASO therapeutics for their applications in the treatment of SDLO diseases. Furthermore, this article is expected to provide new knowledge to scientists and trainees that are unfamiliar with the new generation of therapeutic agents.
Authorship Contributions
Participated in research design: Gogate, Belcourt, Shah, Wang, Frankel, Kolmel, Chalon, Stephen, Kolli, Tawfik, Jin, Bahal, Rasmussen, Manautou, Zhong.
Performed data analysis: Gogate, Belcourt, Shah, Wang, Frankel, Kolmel, Chalon, Stephen, Kolli, Tawfik, Jin, Zhong.
Wrote or contributed to the writing of the manuscript: Gogate, Belcourt, Shah, Wang, Frankel, Kolmel, Chalon, Stephen, Kolli, Tawfik, Jin, Bahal, Rasmussen, Manautou, Zhong.
Footnotes
- Received January 3, 2024.
- Revision received August 25, 2023.
- Accepted September 6, 2023.
This study was supported by National Institutes of Health National Institute of General Medical Sciences [Grant R35GM140862] (to X.Z.), National Heart, Lung, and Blood Institute [Grant R01HL147028] (to R.B.), and an Endowment Fund from Boehringer Ingelheim Pharmaceuticals, Inc. (to J.E.M., who is the Boehringer Ingelheim Pharmaceuticals, Inc. Endowed Chair in Mechanistic Toxicology).
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- 2’-F
- 2’-fluoro
- 2’-MOE
- 2’-O-methyloxyethyl
- 2’-OMe
- 2’-O-methyl
- 3′-UTR
- 3′-untranslated region
- AAT
- α-1 antitrypsin
- AATLD
- α-1 antitrypsin deficiency-associated liver disease
- ACE
- angiotensin-converting enzyme
- ADP
- δ-aminolaevulinic acid dehydratase deficiency porphyria
- ADR
- adverse drug reaction
- AGXT/AGT
- alanine-glyoxylate aminotransferase
- AHP
- acute hepatic porphyria
- aHUS
- atypical hemolytic uremic syndrome
- AIP
- acute intermittent porphyria
- ALA
- aminolaevulinic acid
- ALAD
- ALA dehydrase
- ALAS1
- δ-aminolaevulinic acid synthase 1
- ALT
- alanine transaminase
- APOB
- apolipoprotein B
- ARB
- angiotensin receptor blocker
- ASCVD
- atherosclerotic cardiovascular disease
- ASGPR
- asialoglycoprotein receptor
- ASO
- antisense oligonucleotide
- AST
- aspartate transaminase
- AT
- antithrombin
- C5
- complement 5
- CMD
- complement-mediated disease
- CpG
- cytosine-guanine
- FDA
- Food and Drug Administration
- FH
- familial hypercholesterolemia
- F-IX
- factor IX
- F-VIII
- factor VIII
- GalNAc
- N-acetylgalactosamine
- GO
- glycolate oxidase
- GR
- glyoxylate reductase
- HAO1
- hydroxy acid oxidase 1
- hATTR
- hereditary transthyretin amyloidosis
- HCP
- hereditary coproporphyrin
- HOGA1
- 4-hydroxy-2-oxoglutarate aldolase 1
- IgAN
- IgA nephropathy
- LDL
- low-density lipoprotein
- LDL-C
- LDL cholesterol
- LDL-R
- LDL receptor
- LNP
- lipid nanoparticle
- Lp(a)
- lipoprotein(a)
- miRNA
- microRNA
- NF-κB
- nuclear factor κ-light-chain-enhancer
- PBG
- porphobilinogen
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- PD
- pharmacodynamic
- PDA
- principle of drug action
- PH
- primary hyperoxaluria
- PK
- pharmacokinetic
- PNH
- paroxysmal nocturnal hemoglobinuria
- PS
- phosphorothioate
- REMS
- Risk Evaluation and Mitigation Strategy
- RISC
- RNA-induced silencing complex
- RNAi
- RNA interfering
- SDLO
- systemic disease of liver origin
- SERPINA1
- serine protease inhibitor 1
- siRNA
- small interfering RNA
- TLR
- toll-like receptor
- TTR
- transthyretin
- VP
- variegate porphyria.
- Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics
References
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵