Abstract
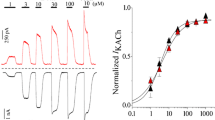
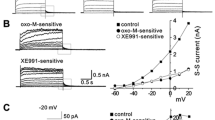
The selective α1-adrenergic agonist methoxamine (10−4–10−3M), in the presence of propranolol (10−6M), can reduce both the inwardly rectifying K+ background current (I K1) and the muscarinic cholinergic receptor-activated K+ current (I K, ACh) in rabbit atrial myocytes resulting in action potential prolongation during the final phase of repolarization and a depolarization of the resting membrane potential. The reduction of these K+ current(s) by α1-adrenoceptor stimulation was insensitive to pre-treatment of artial myocytes with pertussis toxin (0.15–0.5 μg/ml) and was irreversible following intracellular dialysis with the non-hydrolysable guanosine triphosphate (GTP) analogue, Gpp(NH)p (1−5×10−3M). Neither the protein kinase C (PKC) inhibitors, 1-(5-isoquinolinesulphonyl)-2-methylpiperoxine (H-7) (5×10−5M) and staurosporine (1×10−7M), nor “downregulation” of PKC by prolonged phorbol ester exposure (5×10−7M, for 7–8 h) had an effect on the α1-adrenergic modulation of this K+ current. Under cellattached patch-clamp conditions, bath application of methoxamine reversibly decreased acetylcholine-induced single-channel activity, thus confirming the observed reduction of the ACh-induced current under whole-cell voltage clamp. These results demonstrate that the α1adrenoceptor, once activated, can reduce current through two different inwardly rectifying K+ channels in rabbit atrial myocytes. These current changes are mediated via a pertussis toxin-insensitive GTP-binding protein, and do not appear to involve the activation of PKC.
Similar content being viewed by others
References
Bohm M, Schmitz W, Scholz H (1987) Evidence against a role of a pertussis toxin-sensitive guanine nucleotide-binding protein in the alpha1-adrenoceptor-mediated positive inotropic effect in the heart. Naunyn Schmiedeberg's Arch Pharmacol 35:476–479
Braun AP, Fedida D, Clark RB, Giles WR (1990) Intracellular mechanisms for α1-adrenergic regulation of the transient outward current in rabbit atrial myocytes. J Physiol (Lond) 431:689–712
Brown AM, Birnbaumer L (1990) Ionic channels and their regulation by G protein subunits. Annu Rev Physiol 52:197–213
Buxton ILO, Brunton LL (1985) Actions of the cardiac α1-adrenergic receptor. J Biol Chem 26:6733–6737
Clark RB, Nakajima T, Giles W, Kanai K, Momose Y, Szabo G (1990) Two distinct types of inwardly rectifying K+ channels in bull-frog atrial myocytes. J Physiol (Lond) 424:229–251
Endoh M, Schumann HJ (1975) Frequency-dependence of the positive inotropic effect of methoxamine and naphazoline mediated by α-adrenoceptors in the isolated rabbit papillary muscle. Naunyn Schmiedeberg's Arch Pharmacol 287:377–389
Endoh M, Hiramoto T, Kushida H (1989) Preponderance of β-over α-adrenoceptors in mediating the positive inotropic effect of phenylephrine in the ferret myocardium. Naunyn Schmiedeberg's Arch Pharmacol 339:362–366
Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of the solutions containing multiple metals and lignads used for experiments in skinned muscle cells. J Physiol (Paris) 75:463–505
Fedida D, Giles WR (1989) A phorbol ester increases the transient outward current in rabbit atrial myocytes (abstract). J Physiol (Lond) 415:106P
Fedida D, Shimoni Y, Giles WR (1989) A novel effect of norepinephrine on cardiac cells is mediated by α1-adrenoceptors. Am J Physiol 256:H 1500-H 1504
Fedida D, Shimoni Y, Giles WR (1990) α-Adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol (Lond) 423:257–277
Fedida D, Braun AP, Giles WR (1991) α1-Adrenoceptors reduce background K+ current in rabbit ventricular myocytes. J Physiol (Lond) 441:673–684
Gadsby DC, Glitsch HG (1982) Change in membrane current mediated by α-adrenoceptors in canine cardiac Purkinje fibres (abstract). J Physiol (Lond) 332:51 P
Giles WR, Imaizumi Y (1988) Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol (Lond) 405:123–145
Giles WR, Shibata EF (1981) Autonomic transmitter actions on cardiac pacemaker tissue: a brief review. Fed Proc 40:2618–2625
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Hartmann HA, Mazzocca NJ, Kleimann RB, Houser SR (1988) Effects of phenylephrine on calcium current and contractility of feline ventricular myocytes. Am J Physiol 255:H 1173-H 1180
Hartzell HC (1988) Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol 52:165–247
Hauswirth O, Wehner HD, Ziskoven R (1976) α-Adrenergic receptors and pacemaker current in cardiac Purkinje fibres. Nature 263:155–156
Heidbuchel H, Callewaert G, Vereecke J, Carmeliet E (1990) ATPdependent activation of atrial muscarinic K+ channels in the absence of agonist and G-nucleotides. Pflügers Arch. 416:213–215
Jahnel U, Nawrath H, Carmeliet E, Vereecke J (1991) Depolarization-induced influx of sodium in response to phenylephrine in rat atrial heart muscle. J Physiol (Lond) 432:621–637
Kaibara M, Nakajima T, Irisawa H, Giles WR (1991) Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol (Lond) 433:589–613
Kurachi Y, Ito H, Sugimoto T, Shimizu T, Miki I, Ui M (1989) α-Adrenergic activation of the muscarinic K+ channel is mediated by arachidonic acid metabolites. Pflügers Arch 414:102–104
Luetje CW, Tietje KM, Christian JL, Nathanson NM (1988) Differential tissue expression and developmental regulation of guanine nucleotide binding regulatory proteins and thier messenger RNAs in rat heart. J Biol Chem 263:13357–13365
Minneman KP (1988) α-Adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev 40:87–119
Nishizuka Y (1984) The role of protein kinase in cell surface signal transduction and tumour promotion. 308:693–698
Posner P, Farrar EL, Lambert CR (1976) Inhibitory effects of catecholamines in canine cardiac Purkinje fibres. Am J Physiol 231:1415–1420
Ravens U, Wang X-L, Wettwer E (1989) Alpha adrenoceptor stimulation reduces outward currents in rat ventricular myocytes. J Pharmacol Exp Ther 250:364–370
Rosen MR, Hordof AJ, Ilvento JP, Danilo P Jr (1977) Effects of adrenergic amines on electrophysiological properties and automaticity of neonatal and adult canine Purkinje fibers. Circ Res 40:390–400
Rosen MR, Steinberg SF, Chow Y-K, Bilezikian JP, Danilo P Jr (1988) Role of a pertussis toxin-sensitive protein in the modulation of canine Purkinje fiber automaticity. Circ Res 62:315–323
Sakmann B, Noma A, Trautwein W (1983) Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature 303:250–253
Satoh H, Hashimoto K (1988) Effect of α1-adrenoceptor stimulation with methoxamine and phenylephrine on spontaneously beating rabbit sino-atrial node cells. Naunyn Schmiedeberg's Arch Pharmacol 337:415–422
Schmitz W, Scholz H, Scholz J, Steinfath M, Lohse M, Puurunen J, Schwabe U (1987) Pertussis toxin does not inhibit the alpha1-adrenoceptor-mediated effect on inositol phosphate production in the heart. Eur J Pharmacol 134:377–378
Sen L, Liang BT, Colucci WS, Smith TW (1990) Enhanced α1-adrenergic responsiveness in cardiomyopathic hamster cardiac myocytes: Relation to the expression of pertussis toxin-sensitive G protein and α1-adrenergic receptors. Circ Res 67:1182–1192
Shah A, Cohen IS, Rosen MR (1988) Stimulation of cardiac alpha receptors increases Na/K pump current and decreases GK via a pertussis toxin-sensitive pathway. Biophys J 54:219–225
Simmons MA, Hartzell HC (1987) A quantitative analysis of the acetylcholine-activated potassium current in single cells from frog atrium. Pflügers Arch 409:454–461
Steinberg SF, Kaplan LM, Inouye T, Zhang JF, Robinson RB (1989) Alpha-1 adrenergic stimulation of 1,4,5-inositol trisphosphate formation in ventricular myocytes. J Pharmacol Exp Ther 250:1141–1148
Tung L-H, Rand MJ. Louis WJ (1985) Cardiac α-adrenoceptors involving positive chronotropic responses. J Cardiovasc Pharmacol 7 [Suppl6]:S121-S126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braun, A.P., Fedida, D. & Giles, W.R. Activation of α1-adrenoceptors modulates the inwardly rectifying potassium currents of mammalian atrial myocytes. Pflügers Arch 421, 431–439 (1992). https://doi.org/10.1007/BF00370253
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370253