Summary
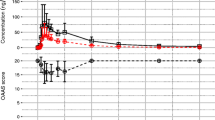
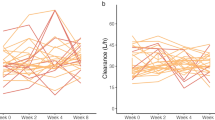
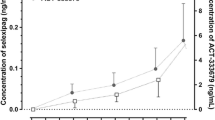
The pharmacokinetics of midazolam and 1-hydroxymethylmidazolam were investigated following oral administration of 7.5, 15 and 30 mg doses of midazolam in solution to 12 healthy subjects. Compared to the 7.5 mg dose, the Cmax and AUC parameters of both midazolam and 1-hydroxymethylmidazolam increased proportionally after the 15 mg dose and more than proportionally after the 30 mg dose. The t1/2 for midazolam remained relatively constant between the 7.5 and 15 mg doses whereas it increased slightly but significantly after the 30 mg dose. These data indicated that the pharmacokinetics of midazolam and 1-hydroxymethylmidazolam were linear between the 7.5 and 15 mg oral dose range. However, after the 30 mg dose, the systemic availability of midazolam and the AUC for 1-hydroxymethylmidazolam appeared to be greater than that anticipated from the lower doses, possibly due to saturation of midazolam first-pass metabolism. This ist not expected to have any clinical significance under the conditions of therapeutic use.
Similar content being viewed by others
References
Allonen H, Ziegler G, Klotz U (1981) Midazolam kinetics. Clin Pharmacol Ther 30: 653–661
Klotz U, Ziegler G (1982) Physiologic and temporal variation in hepatic elimination of midazolam. Clin Pharmacol Ther 32: 107–112
Smith MT, Eadie MJ, O'Rourke Brophy T (1981) The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol 19: 271–278
Heizmann P, Ziegler WH (1981) Excretion and metabolism of 14C-midazolam in humans following oral dosing. Arzneimittel-forsch/Drug Res 31: 2220–2223
Heizmann P, Eckert M, Ziegler WH (1983) Pharmacokinetics and bioavailability of midazolam in man. Br J Clin Pharmacol 16: 43S-49S
Rubio F, Miwa BJ, Garland WA (1982) Determination of midazolam and two metabolites of midazolam in human plasma by gas chromatography-negative chemical-ionization mass spectrometry. J Chromatogr 233: 157–165
Shand DG, Rangno RE (1972) The disposition of propranolol. Pharmacology 7: 159–168
Raaflaub J, Dubach UC (1975) On the pharmacokinetics of phenacetin in man. Europ J Clin Pharmacol 8: 261–265
Jahnchen E, Bechtold H, Kasper W, Kersting F, Just H, Heykants J, Meinertz T (1979) Lorcainide. Clin Pharmacol Ther 26: 187–195
Christophidis N, Vajda FJE, Lucas I, Drummer O, Moon WJ, Louis WJ (1978) Fluorouracil therapy in patients with carcinoma of the large bowel: A pharmacokinetic comparison of various rates and routes of administration. Clinical Pharmacokinetics 3: 330–336
Walle T, Conradi EC, Walle UK, Fagan TC, Gaffney TE (1980) 4-hydroxypropranolol and its glucuronide after single and long-term doses of propranolol. Clin Pharmacol Ther 27: 22–31
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bornemann, L.D., Min, B.H., Crews, T. et al. Dose dependent pharmacokinetics of midazolam. Eur J Clin Pharmacol 29, 91–95 (1985). https://doi.org/10.1007/BF00547375
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00547375