Abstract
The mechanism underlying the regulation of the K-channel by the muscarinic receptor was examined with patch-clamp experiments in atrial cells isolated enzymatically from the rabbit heart. The patch-electrode and the recording chamber were perfused with various solutions while the activity of the K-channels in the membrane-patch was recorded continuously.
-
1)
In the absence of muscarinic agonists, opening of K-channels occurred at a low frequency (basal activity). Application of ACh to the bath did not affect the basal activity, but perfusion of the patch electrode with ACh markedly increased the channel activity in the “cell-attached” patch. Application of oxotremorine, i.e. a specific muscarinic agonist, via the pipette also opened K-channels.
-
2)
When the membrane patch was isolated from the cell body (“inside-out” patch), ACh-induced single K-channel currents were still observed, but the frequency was reduced.
-
3)
Perfusion of atropine or scopolamine, two muscarinic antagonists, through the patch-electrode depressed the basal activity. In the case of scopolamine, channel-activity recovered after washing out the drug.
-
4)
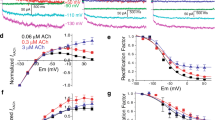
The current voltage relationship determined from the basal activity was similar to that of ACh-induced single K-channel currents.
-
5)
The mean open time was 0.49 ms at basal activity and 1.35 ms during the application of 0.1 μM ACh via the patch electrode. Application of oxotremorine via the pipette hardly affected the open-time, it remained at 99±45 (n=7) of the control.
These results suggest that the ACh-induced K current is generated by a class of K-channels having open and close kinetics and that the ACh-muscarinic receptor-complex directly increases the probability of this K-channel being open by coupling with the channel. The increase in openstate probability is mainly due to shortening of the mean closed-time. Basal activity is most probably generated by this K-channel. The muscarinic antagonists depress the ACh-induced K-current not only by blocking the binding of the agonist to the receptor, but also through a depressing influence of the antagonist-receptor-complex on the K-channel.
Similar content being viewed by others
References
Cuatrecasas P (1974) Membrane receptors. Ann Rev Biochem 43:169–214
Drummond GI, Severson DL (1979) Cyclic nucleotides and cardiac function. Circ Res 44:145–153
Glitsch HG, Pott L (1978) Effects of acetylcholine in parasympathetic nerve stimulation on membrane potential in quiescent guinea-pig atria. J Physiol (Lond) 279:655–668
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improve patch-clamp technique for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hartzell CH (1981) Mechanisms of slow postsynaptic potentials. Nature 291:539–543
Isenberg G, Klöckner U (1982) Calcium tolerant ventricular myocytes prepared by preincubation in a „KB Medium”. Pflügers Arch 395:6–18
Kakei M, Noma A (1984) Adenosine 5′-triphosphate sensitive single potassium channel in the A-V node cell of the rabbit heart. J Physiol (in press)
Kameyama M, Kiyosue T, Soejima M (1984) Single-channel analysis of the inward rectifier current in the rabbit ventricular cells. Jpn J Physiol (in press)
Kehoe JS, Marty A (1980) Certain slow synaptic responses: Their properties and possible underlying mechanisms. Ann Rev Biophys Bioeng 9:437–465
Maruyama Y, Petersen OH (1982) Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature 300:61–63
Nawrath H (1977) Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature 267:72–74
Neher E, Sakmann B (1976) Single channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802
Noma A, Kokubun S, Taniguchi J (1981) Ionic mechanism underlying muscarinic acetylcholine response in the rabbit sinoatrial node. J Physiol (Paris) 77:1073–1076
Noma A, Osterrieder W, Trautwein W (1979b) The effect of external potassium on the elementary conductance of the ACh-induced potassium channel in the sino-atrial node. Pflügers Arch 381:263–269
Noma A, Peper K, Trautwein W (1979a) Acetylcholine-induced potassium current fluctuations in the rabbit sino-atrial node. Pflügers Arch 381:255–262
Noma A, Trautwein W (1978) Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflügers Arch 377:193–200
Noma A (1983) ATP-regulated single K channels in cardiac muscle. Nature 305:147–148
Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F (1982) Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298:576–578
Osterrieder W, Noma A, Trautwein W (1980) On the kinetics of the potassium channel activated by acetylcholine in the S-A node of the rabbit heart. Pflügers Arch 386:101–109
Osterrieder W, Yang QF, Trautwein W (1981) The time course of the muscarinic response to ionophoretic acetylcholine application to the S-A node of the rabbit heart. Pflügers Arch 389:283–291
Pott L (1979) On the time course of the acetylcholine-induced hyperpolarization in quiescent guinea pig atria. Pflügers Arch 380:71–77
Powell T, Twist VW (1976) A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Comm 72:327–333
Reuter H, Stevens CF, Tsien RW, Yellen G (1982) Properties of single calcium channel in cardiac cell culture. Nature 297:501–504
Sakmann B, Noma A, Trautwein W (1983) Acetylcholine activation of single muscarinic K channels in isolated pacemaker cells of the mammalian heart. Nature 303:250–253
Siegelbaum SA, Camardo JS, Kandel ER (1982) Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature 299:413–417
Taniguchi J, Kokubun S, Noma A, Irisawa H (1981) Spontaneously active cells isolated from the sino-atrial and atrio-ventricular node of the rabbit heart. Jpn J Physiol 31:547–558
Trautwein W, Tamiguchi J, Noma A (1982) The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflügers Arch 392:307–314
Trube G, Sakmann B, Trautwein W (1981) Inward rectifying potassium currents recorded from isolated heart cells by the patch clamp method. Pflügers Arch 391:[Suppl] R 7
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soejima, M., Noma, A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 400, 424–431 (1984). https://doi.org/10.1007/BF00587544
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00587544