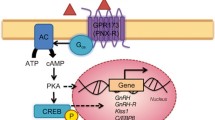
Abstract
Since its discovery in the 1920s, relaxin has enjoyed a reputation as a peptide hormone of pregnancy. However, relaxin and other relaxin family peptides are now associated with numerous non-reproductive physiologies and disease states. The new millennium bought with it the sequence of the human genome and subsequently new directions for relaxin research. In 2002, the ancestral relaxin gene RLN3 was identified from genome databases. The relaxin-3 peptide is highly expressed in a small region of the brain and in species from teleost to primates and has both conserved sequence and sites of expression. Combined with the discovery of the relaxin family peptide receptors, interest in the role of the relaxin family peptides in the central nervous system has been reignited. This review explores the relaxin family peptides that are expressed in or act upon the brain, the receptors that mediate their actions, and what is currently known of their functions.







Similar content being viewed by others
References
Hisaw F (1926) Experimental relaxation of the pubic ligament of the guinea pig. Proc Soc Exp Biol Med 23:661–663
Fevold HL, Hisaw FL, Meyer RK (1930) The relaxative hormone of the corpus luteum: its purification and concentration. J Am Chem Soc 52:3340–3348
Sherwood CD, O’Byrne EM (1974) Purification and characterization of porcine relaxin. Arch Biochem Biophys 160:185–196
James R, Niall H, Kwok S, Bryand-Greenwood G (1977) Primary structure of porcine relaxin: homology with insulin and related growth factors. Nature 267:544–546
Bullesbach EE, Schwabe C (2000) The relaxin receptor-binding site geometry suggests a novel gripping mode of interaction. J Biol Chem 275:35276–35280
Hudson P, Haley J, John M, Cronk M, Crawford R, Haralambidis J, Tregear G, Shine J, Niall H (1983) Structure of a genomic clone encoding biologically active human relaxin. Nature 301:628–631
Hudson P, John M, Crawford R, Haralambidis J, Scanlon D, Gorman J, Tregear G, Shine J, Niall H (1984) Relaxin gene expression in human ovaries and the predicted structure of a human preprorelaxin by analysis of cDNA clones. EMBO J 3:2333–2339
Drolet DW, Henzel WJ, Johnston PD (1987) Purification, amino-terminal sequencing and demonstration of biological activity of human relaxin from corpora lutea. In: Program of the 69th Annual Meeting of the Endocrine Society, p 196 (abstract), Indianapolis
Winslow J, Shih A, Laramee G, Bourell J, Stults J, Johnston P (1989) Purification and structure of human pregnancy relaxin from corpora lutea, serum and plasma. In: Program of the 71st Annual Meeting of the Endocrine Society, p 245 (abstract), Seattle
Adham IM, Burkhardt E, Benahmed M, Engel W (1993) Cloning of a cDNA for a novel insulin-like peptide of the testicular Leydig cells. J Biol Chem 268:26668–26672
Chassin D, Laurent A, Janneau JL, Berger R, Bellet D (1995) Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics 29:465–470
Conklin D, Lofton-Day CE, Haldeman BA, Ching A, Whitmore TE, Lok S, Jaspers S (1999) Identification of INSL5, a new member of the insulin superfamily. Genomics 60:50–56
Lok S, Johnston DS, Conklin D, Lofton-Day CE, Adams RL, Jelmberg AC, Whitmore TE, Schrader S, Griswold MD, Jaspers SR (2000) Identification of INSL6, a new member of the insulin family that is expressed in the testis of the human and rat. Biol Reprod 62:1593–1599
Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG, Dawson NF, Zhao C, Bond C, Summers RJ, Parry LJ, Wade JD, Tregear GW (2002) Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem 277:1148–1157
Wilkinson TN, Speed TP, Tregear GW, Bathgate RA (2005) Evolution of the relaxin-like peptide family. BMC Evol Biol 5:14
Wilkinson TN, Speed TP, Tregear GW, Bathgate RA (2005) Coevolution of the relaxin-like peptides and their receptors. Ann NY Acad Sci 1041:534–539
Bathgate RA, Hsueh AJ, Sherwood OD (2005) Physiology and molecular biology of the relaxin peptide family. In: Neil JD (ed) Physiology of reproduction. Elsevier, San Diego, pp 679–768
Samuel CS, Tian H, Zhao L, Amento EP (2003) Relaxin is a key mediator of prostate growth and male reproductive tract development. Lab Invest 83:1055–1067
Weiss G (1989) Relaxin in the male. Biol Reprod 40:197–200
Hsu S, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood O, Hsueh A (2002) Activation of orphan receptors by the hormone relaxin. Science 295:671–674
Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F (1999) Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology 140:445–453
Krajnc-Franken MAM, van Disseldorp AJM, Koenders JE, Mosselman S, van Duin M, Gossen JA (2004) Impaired nipple development and parturition in LGR7 knockout mice. Mol Cell Biol 24:687–696
Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272
Bullesbach EE, Schwabe C (2005) The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J Biol Chem 280:14051–14056
Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJW, Tregear GW, Bathgate RAD (2006) Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem 281:34942–34954
Hopkins EJ, Layfield S, Ferraro T, Bathgate RAD, Gooley PR (2007) The NMR solution structure of the relaxin (RXFP1) receptor lipoprotein receptor class A module and identification of key residues in the N-terminal region of the module that mediate receptor activation. J Biol Chem 282:4172–4184
Ivell R, Anand-Ivell R, Bartsch O (2005) Relaxin signaling from natural receptors. Ann NY Acad Sci 1041:280–287
Halls ML, van der Westhuizen ET, Bathgate RA, Summers RJ (2007) Relaxin family peptide receptors–former orphans reunite with their parent ligands to activate multiple signalling pathways. Br J Pharmacol 150:677–691
Nef S, Parada LF (1999) Cryptorchidism in mice mutant for Insl3. Nat Genet 22:295–299
Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM (1999) Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13:681–691
Irving-Rodgers HF, Bathgate RA, Ivell R, Domagalski R, Rodgers RJ (2002) Dynamic changes in the expression of relaxin-like factor (INSL3), cholesterol side-chain cleavage cytochrome p450, and 3beta-hydroxysteroid dehydrogenase in bovine ovarian follicles during growth and atresia. Biol Reprod 66:934–943
Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, Toppari J, Fu P, Wade JD, Bathgate RA, Hsueh AJ (2004) Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA 101:7323–7328
Gorlov IP, Kamat A, Bogatcheva NV, Jones E, Lamb DJ, Truong A, Bishop CE, McElreavey K, Agoulnik AI (2002) Mutations of the GREAT gene cause cryptorchidism. Hum Mol Genet 11:2309–2318
Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ (2002) INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem 277:31283–31286
Scott DJ, Fu P, Shen PJ, Gundlach A, Layfield S, Riesewijk A, Tomiyama H, Hutson JM, Tregear GW, Bathgate RA (2005) Characterization of the Rat INSL3 Receptor. Ann NY Acad Sci 1041:13–16
Bathgate RAD, Lin F, Hanson NF, Otvos L Jr, Guidolin A, Giannakis C, Bastiras S, Layfield SL, Ferraro T, Ma S, Zhao C, Gundlach AL, Samuel CS, Tregear GW, Wade JD (2006) Relaxin-3: Improved synthesis strategy and demonstration of its high affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry 45:1043–1053
Tashima LS, Hieber AD, Greenwood FC, Bryant-Greenwood GD (1995) The human Leydig insulin-like (hLEY I-L) gene is expressed in the corpus luteum and trophoblast. J Clin Endocrinol Metab 80:707–710
Hombach-Klonisch S, Buchmann J, Sarun S, Fischer B, Klonisch T (2000) Relaxin-like factor (RLF) is differentially expressed in the normal and neoplastic human mammary gland. Cancer 89:2161–2168
Zarreh-Hoshyari-Khah MR, Einspanier A, Ivell R (1999) Differential splicing and expression of the relaxin-like factor gene in reproductive tissues of the marmoset monkey (Callithrix jacchus). Biol Reprod 60:445–453
Zimmermann S, Schottler P, Engel W, Adham IM (1997) Mouse Leydig insulin-like (Ley I-L) gene: structure and expression during testis and ovary development. Mol Reprod Dev 47:30–38
Bathgate R, Balvers M, Hunt N, Ivell R (1996) Relaxin-like factor gene is highly expressed in the bovine ovary of the cycle and pregnancy: sequence and messenger ribonucleic acid analysis. Biol Reprod 55:1452–1457
Roche PJ, Butkus A, Wintour EM, Tregear G (1996) Structure and expression of Leydig insulin-like peptide mRNA in the sheep. Mol Cell Endocrinol 121:171–177
Burazin TC, Bathgate RA, Macris M, Layfield S, Gundlach AL, Tregear GW (2002) Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem 82:1553–1557
Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, Hsueh AJ (2003) H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem 278:7855–7862
Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jornvall H, Sillard R, Lovenberg T (2003) Identification of relaxin-3/Insl7 as an endogenous ligand for the orphan G-protein coupled receptor GPCR135. J Biol Chem 278:50754–50764
Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, Wu J, Nepomuceno D, Wilkinson T, Bathgate R, Eriste E, Sillard R, Lovenberg TW (2005) INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J Biol Chem 280:292–300
Liu C, Chen J, Kuei C, Sutton S, Nepomuceno D, Bonaventure P, Lovenberg TW (2005) Relaxin-3/insulin-like peptide 5 chimeric peptide, a selective ligand for G protein-coupled receptor (GPCR)135 and GPCR142 over leucine-rich repeat-containing G protein-coupled receptor 7. Mol Pharmacol 67:231–240
Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA, Liu C, Tregear GW, Sutton SW, Gundlach AL (2007) Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience 144:165–190
Sutton SW, Bonaventure P, Kuei C, Roland B, Chen J, Nepomuceno D, Lovenberg TW, Liu C (2004) Distribution of G-protein-coupled receptor (GPCR)135 binding sites and receptor mRNA in the rat brain suggests a role for relaxin-3 in neuroendocrine and sensory processing. Neuroendocrinology 80:298–307
Shen PJ, Fu P, Phelan KD, Scott DJ, Layfield S, Tregear GW, Bathgate RA, Gundlach AL (2005) Restricted expression of LGR8 in intralaminar thalamic nuclei of rat brain suggests a role in sensorimotor systems. Ann NY Acad Sci 1041:510–515
Sedaghat K, Shen PJ, Finkelstein DI, Henderson JM, Gundlach AL (2008) Leucine-rich repeat-containing G-protein-coupled receptor 8 in the rat brain: enrichment in thalamic neurons and their efferent projections. Neuroscience 156:319–333
Bathgate R, Moniac N, Bartlick B, Schumacher M, Fields M, Ivell R (1999) Expression and regulation of relaxin-like factor gene transcripts in the bovine ovary: differentiation-dependent expression in theca cell cultures. Biol Reprod 61:1090–1098
Anand-Ivell R, Heng K, Hafen B, Setchell B, Ivell R (2009) Dynamics of INSL3 peptide expression in the rodent testis. Biol Reprod 81:480–487
Foresta C, Bettella A, Vinanzi C, Dabrilli P, Meriggiola MC, Garolla A, Ferlin A (2004) A novel circulating hormone of testis origin in humans. J Clin Endocrinol Metab 89:5952–5958
Osheroff PL, Ho WH (1993) Expression of relaxin mRNA and relaxin receptors in postnatal and adult rat brains and hearts. Localization and developmental patterns. J Biol Chem 268:15193–15199
Gunnersen JM, Crawford RJ, Tregear GW (1995) Expression of the relaxin gene in rat tissues. Mol Cell Endocrinol 110:55–64
Ma S, Roozendaal B, Burazin TC, Tregear GW, McGaugh JL, Gundlach AL (2005) Relaxin receptor activation in the basolateral amygdala impairs memory consolidation. Eur J Neurosci 22:2117–2122
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176
Osheroff PL, Ling VT, Vandlen RL, Cronin MJ, Lofgren JA (1990) Preparation of biologically active 32P-labeled human relaxin. Displaceable binding to rat uterus, cervix, and brain. J Biol Chem 265:9396–9401
Osheroff PL, Phillips HS (1991) Autoradiographic localization of relaxin binding sites in rat brain. Proc Natl Acad Sci USA 88:6413–6417
Ma S, Shen PJ, Burazin TC, Tregear GW, Gundlach AL (2006) Comparative localization of leucine-rich repeat-containing G-protein-coupled receptor-7 (RXFP1) mRNA and [(33)P]-relaxin binding sites in rat brain: restricted somatic co-expression a clue to relaxin action? Neuroscience 141:329–344
Summerlee AJ, O’Byrne KT, Jones SA, Eltringham L (1987) The subfornical organ and relaxin-induced inhibition of reflex milk ejection in lactating rats. J Endocrinol 115:347–353
O’Byrne KT, Eltringham L, Summerlee AJ (1987) Central inhibitory effects of relaxin on the milk ejection reflex of the rat depends upon the site of injection into the cerebroventricular system. Brain Res 405:80–83
Mumford AD, Parry LJ, Summerlee AJ (1989) Lesion of the subfornical organ affects the haemotensive response to centrally administered relaxin in anaesthetized rats. J Endocrinol 122:747–755
Swanson LW, Sawchenko PE (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
Summerlee AJ, O’Byrne KT, Paisley AC, Breeze MF, Porter DG (1984) Relaxin affects the central control of oxytocin release. Nature 309:372–374
Dayanithi G, Cazalis M, Nordmann JJ (1987) Relaxin affects the release of oxytocin and vasopressin from the neurohypophysis. Nature 325:813–816
Parry LJ, Summerlee AJ (1991) Central angiotensin partially mediates the pressor action of relaxin in anesthetized rats. Endocrinology 129:47–52
Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S (1982) Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol 330:81–93
Summerlee AJ, Hornsby DJ, Ramsey DG (1998) The dipsogenic effects of rat relaxin: the effect of photoperiod and the potential role of relaxin on drinking in pregnancy. Endocrinology 139:2322–2328
Sunn N, McKinley MJ, Oldfield BJ (2001) Identification of efferent neural pathways from the lamina terminalis activated by blood-borne relaxin. J Neuroendocrinol 13:432–437
Sunn N, Egli M, Burazin TC, Burns P, Colvill L, Davern P, Denton DA, Oldfield BJ, Weisinger RS, Rauch M, Schmid HA, McKinley MJ (2002) Circulating relaxin acts on subfornical organ neurons to stimulate water drinking in the rat. Proc Natl Acad Sci USA 99:1701–1706
Sinnayah P, Burns P, Wade JD, Weisinger RS, McKinley MJ (1999) Water drinking in rats resulting from intravenous relaxin and its modification by other dipsogenic factors. Endocrinology 140:5082–5086
Geddes BJ, Parry LJ, Summerlee AJ (1994) Brain angiotensin-II partially mediates the effects of relaxin on vasopressin and oxytocin release in anesthetized rats. Endocrinology 134:1188–1192
Guico-Lamm ML, Sherwood OD (1988) Monoclonal antibodies specific for rat relaxin. II. Passive immunization with monoclonal antibodies throughout the second half of pregnancy disrupts birth in intact rats. Endocrinology 123:2479–2485
Summerlee AJ, Ramsey DG, Poterski RS (1998) Neutralization of relaxin within the brain affects the timing of birth in rats. Endocrinology 139:479–484
McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28
Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y (2005) Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21:1659–1670
Morest DK (1961) Connexions of the dorsal tegmental nucleus in rat and rabbit. J Anat 95:229–246
Jennes L, Beckman WC, Stumpf WE, Grzanna R (1982) Anatomical relationships of serotoninergic and noradrenalinergic projections with the GnRH system in septum and hypothalamus. Exp Brain Res 46:331–338
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Smith CM, Shen PJ, Ma S, Sutton SW, Gundlach AL (2009) Verification of a relaxin-3 knockout/LacZ reporter mouse as a model of relaxin-3 deficiency. Ann NY Acad Sci 1160:259–260
Donizetti A, Grossi M, Pariante P, D’Aniello E, Izzo G, Minucci S, Aniello F (2008) Two neuron clusters in the stem of postembryonic zebrafish brain specifically express relaxin-3 gene: first evidence of nucleus incertus in fish. Dev Dyn 237:3864–3869
Ma S, Sang Q, Lanciego JL, Gundlach AL (2009) Localization of relaxin-3 in brain of Macaca fascicularis: identification of a nucleus incertus in primate. J Comp Neurol 517:856–872
Silvertown JD, Neschadim A, Liu HN, Shannon P, Walia JS, Kao JC, Robertson J, Summerlee AJ, Medin JA (2009) Relaxin-3 and receptors in the human and rhesus brain and reproductive tissues. Regul Pept 159:44–53
Goto M, Swanson LW, Canteras NS (2001) Connections of the nucleus incertus. J Comp Neurol 438:86–122
Ford B, Holmes CJ, Mainville L, Jones BE (1995) GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol 363:177–196
Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F (2003) Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol 464:62–97
Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W (1994) Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA 91:8777–8781
Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W (1982) Corticotropin releasing factor produces behavioural activation in rats. Nature 297:331–333
Banerjee A, Shen PJ, Ma S, Bathgate RA, Gundlach AL (2009) Swim stress excitation of nucleus incertus and rapid induction of relaxin-3 expression via CRF(1) activation. Neuropharmacology 58:145–155
McGowan BM, Stanley SA, Smith KL, White NE, Connolly MM, Thompson EL, Gardiner JV, Murphy KG, Ghatei MA, Bloom SR (2005) Central relaxin-3 administration causes hyperphagia in male Wistar rats. Endocrinology 146:3295–3300
McGowan BM, Stanley SA, White NE, Spangeus A, Patterson M, Thompson EL, Smith KL, Donovan J, Gardiner JV, Ghatei MA, Bloom SR (2007) Hypothalamic mapping of orexigenic action and Fos-like immunoreactivity following relaxin-3 administration in male Wistar rats. Am J Physiol Endocrinol Metab 292:E913–E919
McGowan BM, Stanley SA, Smith KL, Minnion JS, Donovan J, Thompson EL, Patterson M, Connolly MM, Abbott CR, Small CJ, Gardiner JV, Ghatei MA, Bloom SR (2006) Effects of acute and chronic relaxin-3 on food intake and energy expenditure in rats. Regul Pept 136:72–77
Hida T, Takahashi E, Shikata K, Hirohashi T, Sawai T, Seiki T, Tanaka H, Kawai T, Ito O, Arai T, Yokoi A, Hirakawa T, Ogura H, Nagasu T, Miyamoto N, Kuromitsu J (2006) Chronic intracerebroventricular administration of relaxin-3 increases body weight in rats. J Recept Signal Transduct Res 26:147–158
Cools R, Roberts AC, Robbins TW (2008) Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12:31–40
Miyamoto Y, Watanabe Y, Tanaka M (2008) Developmental expression and serotonergic regulation of relaxin 3/INSL7 in the nucleus incertus of rat brain. Regul Pept 145:54–59
Nunez A, Cervera-Ferri A, Olucha-Bordonau F, Ruiz-Torner A, Teruel V (2006) Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci 23:2731–2738
Ma S, Olucha-Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C, Wade JD, Sutton SW, Nunez A, Gundlach AL (2009) Modulation of hippocampal theta oscillations and spatial memory by relaxin-3 neurons of the nucleus incertus. Learn Mem 16:730–742
Kuei C, Sutton S, Bonaventure P, Pudiak C, Shelton J, Zhu J, Nepomuceno D, Wu J, Chen J, Kamme F, Seierstad M, Hack MD, Bathgate RA, Hossain MA, Wade JD, Atack J, Lovenberg TW, Liu C (2007) R3(BDelta23–27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization. J Biol Chem 282:25425–25435
Callander GE, Thomas WG, Bathgate RA (2009) Development and optimization of microRNA against relaxin-3. Ann NY Acad Sci 1160:261–264
Acknowledgments
Gabrielle Callander is supported by a University of Melbourne Postgraduate Research Scholarship and Ross Bathgate by an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellowship. We wish to thank Dr. Sherie Ma and A/Prof Andrew Gundlach for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Callander, G.E., Bathgate, R.A.D. Relaxin family peptide systems and the central nervous system. Cell. Mol. Life Sci. 67, 2327–2341 (2010). https://doi.org/10.1007/s00018-010-0304-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0304-z