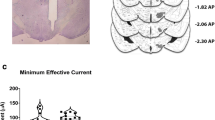
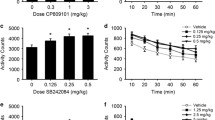
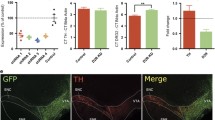
Abstract
Rationale
The rewarding effects of lateral hypothalamic brain stimulation, various natural rewards, and several drugs of abuse are attenuated by D1 or D2 dopamine receptor (D1R or D2R) antagonists. Much of the evidence for dopaminergic involvement in rewards is based on pharmacological agents with limited or “relative” selectivity for dopamine receptor subtypes. Genetically engineered animal models provide a complementary approach to pharmacological investigations.
Objectives
In the present study, we explored the contribution of dopamine D2Rs to (1) brain stimulation reward (BSR) and (2) the potentiation of this behavior by morphine and amphetamine using D2R-deficient mice.
Methods
Wild-type (D2Rwt), heterozygous (D2Rhet), and D2R knockout (D2Rko) mice were trained to turn a wheel for rewarding brain stimulation. Once equivalent rate–frequency curves were established, morphine-induced (0, 1.0, 3.0, and 5.6 mg/kg s.c.) and amphetamine-induced (0, 1.0, 2.0, and 4.0 mg/kg i.p.) potentiations of BSR were determined.
Results
The D2Rko mice required approximately 50% more stimulation than the D2Rwt mice did. With the equi-rewarding levels of stimulation current, amphetamine potentiated BSR equally across the three genotypes. In contrast, morphine potentiated rewarding stimulation in the D2Rwt, had no effect in the D2Rhet, and antagonized rewarding stimulation in the D2Rko mice.
Conclusions
D2R elimination decreases, but does not eliminate, the rewarding effects of lateral hypothalamic stimulation. After compensation for this deficit, amphetamine continues to potentiate BSR, while morphine does not.







Similar content being viewed by others
References
Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ (1998) Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol 343:111–118
Alderson HL, Parkinson JA, Robbins TW, Everitt BJ (2001) The effects of excitotoxic lesions of the nucleus accumbens core or shell regions on intravenous heroin self-administration in rats. Psychopharmacology (Berl) 153:455–463
Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377:424–428
Benoit-Marand M, Borrelli E, Gonon F (2001) Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci 21:9134–9141
Bozzi Y, Borrelli E (1999) Absence of the dopamine D2 receptor leads to a decreased expression of GDNF and NT-4 mRNAs in restricted brain areas. Eur J Neurosci 11:1275–1284
Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E (2002) Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22:2977–2988
Cameron DL, Williams JT (1994) Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci 14:6763–6767
Carlezon WA Jr, Devine DP, Wise RA (1995) Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 122:194–197
Carlson JH, Bergstrom DA, Walters JR (1987) Stimulation of both D1 and D2 dopamine receptors appears necessary for full expression of postsynaptic effects of dopamine agonists: a neurophysiological study. Brain Res 400:205–218
Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS (2001) Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol 85:659–670
Chausmer AL, Katz JL (2001) The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology (Berl) 155:69–77
Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL (2002) Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 163:54–61
Clark D, White FJ (1987) D1 dopamine receptor—the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse 1:347–388
David V, Cazala P (2000) Anatomical and pharmacological specificity of the rewarding effect elicited by microinjections of morphine into the nucleus accumbens of mice. Psychopharmacology (Berl) 150:24–34
David V, Durkin TP, Cazala P (2002) Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology (Berl) 160:307–317
Devine DP, Wise RA (1994) Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci 14:1978–1984
Devine DP, Leone P, Pocock D, Wise RA (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266:1236–1246
De Wit H, Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol 31:195–203
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR (1999) Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem 72:148–156
Dockstader CL, Rubinstein M, Grandy DK, Low MJ, van der Kooy D (2001) The D2 receptor is critical in mediating opiate motivation only in opiate-dependent and withdrawn mice. Eur J Neurosci 13:995–1001
Drago F, Contarino A, Busa L (1999) The expression of neuropeptide-induced excessive grooming behavior in dopamine D1 and D2 receptor-deficient mice. Eur J Pharmacol 365:125–131
Edmonds DE, Stellar JR, Gallistel CR (1974) Parametric analysis of brain stimulation reward in the rat. II. Temporal summation in the reward system. J Comp Physiol Psychol 87:860–869
Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA (2002) Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci 22(RC224):1–6
Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 78:204–209
Fischer JF, Cho AK (1979) Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther 208:203–209
Fouriezos G, Wise RA (1976) Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res 103:377–380
Fouriezos G, Hansson P, Wise RA (1978) Neuroleptic-induced attenuation of brain stimulation reward in rats. J Comp Physiol Psychol 92:661–671
Franklin KB, McCoy SN (1979) Pimozide-induced extinction in rats: stimulus control of responding rules out motor deficit. Pharmacol Biochem Behav 11:71–75
Gallistel CR (1987) Determining the quantitative characteristics of a reward pathway. In: Church RM, Commons ML, Stellar JR, Wagner AR (eds) Biological determinants of reinforcement. Lawrence Erlbaum Associates, Hillsdale, NJ, pp 1–30
Gallistel CR, Karras D (1984) Pimozide and amphetamine have opposing effects on the reward summation function. Pharmacol Biochem Behav 20:73–77
Gallistel CR, Freyd G (1987) Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav 26:731–741
Gilliss B, Malanga CJ, Pieper JO, Carlezon WA Jr (2002) Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss–Webster mice. Psychopharmacology (Berl) 163:238–248
Goeders NE, Lane JD, Smith JE (1984) Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol Biochem Behav 20:451–455
Grech DM, Spealman RD, Bergman J (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 125:97–104
Groves PM, Wilson CJ, Young SJ, Rebec GV (1975) Self-inhibition by dopaminergic neurons. Science 190:522–528
Hand TH, Franklin KB (1985) 6-OHDA lesions of the ventral tegmental area block morphine-induced but not amphetamine-induced facilitation of self-stimulation. Brain Res 328:233–241
Hayward MD, Low MJ (in press) Naloxonev's suppression of spontaneous and food conditioned locomotor activity is diminished in mice lacking either the dopamine D2 receptor or enkephalin. Mol Brain Res
Heikkila RE, Orlansky H, Cohen G (1975) Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol 24:847–852
Henry DJ, Wise RA, Rompre PP, White FJ (1992) Acute depolarization block of A10 dopamine neurons: interactions of morphine with dopamine antagonists. Brain Res 596:231–237
Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS (2004) Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci 19:2455–2463
Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U (1989) Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res 498:199–203
Hutcheson DM, Parkinson JA, Robbins TW, Everitt BJ (2001) The effects of nucleus accumbens core and shell lesions on intravenous heroin self-administration and the acquisition of drug-seeking behaviour under a second-order schedule of heroin reinforcement. Psychopharmacology (Berl) 153:464–472
Jenck F, Gratton A, Wise RA (1987) Opioid receptor subtypes associated with ventral tegmental facilitation of lateral hypothalamic brain stimulation reward. Brain Res 423:34–38
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ Jr (1983) Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther 227:229–237
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113
Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18:3470–3479
Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem 278:12070–12077
King MA, Bradshaw S, Chang AH, Pintar JE, Pasternak GW (2001) Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: evidence for a tonically active anti-opioid system. J Neurosci 21:7788–7792
Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D (2004) Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci 7:160–169
Loh EA, Roberts DC (1990) Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 101:262–266
Lyness WH, Friedle NM, Moore KE (1980) Increased self-administration of d-amphetamine after destruction of 5-hydroxytryptaminergic neurons. Pharmacol Biochem Behav 12:937–941
Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E (1997) Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature 388:586–589
Murer MG, Dziewczapolski G, Salin P, Vila M, Tseng KY, Ruberg M, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Hirsch E, Raisman-Vozari R, Gershanik O (2000) The indirect basal ganglia pathway in dopamine D(2) receptor-deficient mice. Neuroscience 99:643–650
Nakajima S, Baker JD (1989) Effects of D2 dopamine receptor blockade with raclopride on intracranial self-stimulation and food-reinforced operant behaviour. Psychopharmacology (Berl) 98:330–333
Nakajima S, Patterson RL (1997) The involvement of dopamine D2 receptors, but not D3 or D4 receptors, in the rewarding effect of brain stimulation in the rat. Brain Res 760:74–79
Narita M, Mizuo K, Mizoguchi H, Sakata M, Tseng LF, Suzuki T (2003) Molecular evidence for the functional role of dopamine D3 receptor in the morphine-induced rewarding effect and hyperlocomotion. J Neurosci 23:1006–1012
Olds ME (1982) Reinforcing effects of morphine in the nucleus accumbens. Brain Res 237:429–440
Pettit HO, Justice JB Jr (1989) Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav 34:899–904
Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA (2002) Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci 22:9604–9611
Ralph-Williams RJ, Lehmann-Masten V, Geyer MA (2003) Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology 28:108–118
Ranaldi R, Wise RA (2001) Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 21:5841–5846
Ranck JB Jr (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98:417–440
Roberts DC, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620
Rompre PP, Wise RA (1989a) Behavioral evidence for midbrain dopamine depolarization inactivation. Brain Res 477:152–156
Rompre PP, Wise RA (1989b) Opioid-neuroleptic interaction in brainstem self-stimulation. Brain Res 477:144–151
Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E (2002) Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci 22:3293–3301
Self DW, Stein L (1992) The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 582:349–352
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001) Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci 21:5916–5924
Schmitz Y, Schmauss C, Sulzer D (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci 22:8002–8009
Smith JB, Tetsko LA, Xu R, Wang Y (2002) Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience 113:755–765
Spealman RD, Bergman J, Madras BK, Melia KF (1991) Discriminative stimulus effects of cocaine in squirrel monkeys: involvement of dopamine receptor subtypes. J Pharmacol Exp Ther 258:945–953
Tepper JM, Martin LP, Anderson DR (1995) GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci 15:3092–3103
Weed MR, Woolverton WL (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374
White NM, Packard MG, Hiroi N (1991) Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology (Berl) 103:271–276
Wise RA (1972) Spread of current from monopolar stimulation of the lateral hypothalamus. Am J Physiol 223:545–548
Wise RA (1980) The dopamine synapse and the notion of “pleasure centers” in the brain. Trends Neurosci 3:91–94
Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5:39–87
Wise RA (1989) Opiate reward: sites and substrates. Neurosci Biobehav Rev 13:129–133
Wise RA (1996) Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19:319–340
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Wise RA, Leone P, Rivest R, Leeb K (1995a) Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse 21:140–148
Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB Jr (1995b) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 120:10–20
Woolverton WL (1986) Effects of a D1 and a D2 dopamine antagonist on the self-administration of cocaine and piribedil by rhesus monkeys. Pharmacol Biochem Behav 24:531–535
Xu M, Koeltzow TE, Cooper DC, Tonegawa S, White FJ (1999) Dopamine D3 receptor mutant and wild-type mice exhibit identical responses to putative D3 receptor-selective agonists and antagonists. Synapse 31:210–215
Yeomans JS (1975) Quantitative measurement of neural post-stimulation excitability with behavioral methods. Physiol Behav 15:593–602
Yeomans JS (1995) Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology 12:3–16
Yeomans J, Mercouris N, Ellard C (1985) Behaviorally measured refractory periods are lengthened by reducing electrode tip exposure or raising current. Behav Neurosci 99:913–928
Yeomans JS, Maidment NT, Bunney BS (1988) Excitability properties of medial forebrain bundle axons of A9 and A10 dopamine cells. Brain Res 450:86–93
Yeomans JS, Mathur A, Tampakeras M (1993) Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci 107:1077–1087
Yokel RA, Wise RA (1975) Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science 187:547–549
Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, Larson GA, Kelly MA, Grandy DK, Rubinstein M, Low MJ, Fredholm BB (2000) Functional uncoupling of adenosine A(2A) receptors and reduced response to caffeine in mice lacking dopamine D2 receptors. J Neurosci 20:5949–5957
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elmer, G.I., Pieper, J.O., Levy, J. et al. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology 182, 33–44 (2005). https://doi.org/10.1007/s00213-005-0051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0051-2