Abstract
Rational
A growing body of evidence illustrates that 5-HT3 receptor antagonist drugs may be of benefit in the treatment of negative symptoms in schizophrenia.
Objective
The objective of this study was to assess the efficacy and tolerability of tropisetron add-on to risperidone on negative symptoms in patients with chronic stable schizophrenia.
Methods
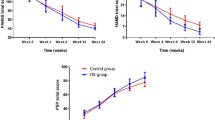
In a double-blind, placebo-controlled 8-week trial, 40 patients with chronic schizophrenia who were stabilized on risperidone were randomized into tropisetron or placebo add-on groups. Psychotic symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) every 2 weeks. Furthermore, extrapyramidal and depressive symptoms as well as side effects were assessed. The primary outcome measure was the difference in change from baseline of negative subscale scores between the two groups at week 8.
Results
Tropisetron resulted in greater improvement of the total PANSS scores [F(1.860,70.699) = 37.366, p < 0.001] as well as negative scores [F(2.439,92.675) = 16.623, p < 0.001] and general psychopathology [F(1.767,67.158) = 4.602, p = 0.017], but not positive subscale scores [F(1.348, 51.218) = 0.048, p = 0.893] compared to placebo. In a multiple regression analysis controlling for positive, extrapyramidal, and depressive symptoms, treatment group (standardized β = −0.640) significantly predicted changes in primary negative symptoms. The side effect profile did not differ significantly between the two groups.
Conclusion
Tropisetron add-on to risperidone improves the primary negative symptoms of patients with chronic stable schizophrenia.





Similar content being viewed by others
References
Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R (1982) Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 17:639–654
Akhondzadeh S (2001) The 5-HT hypothesis of schizophrenia. IDrugs 4:295–300
Akhondzadeh S, Malek-Hosseini M, Ghoreishi A, Raznahan M, Rezazadeh SA (2008) Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 32:1879–1883
Akhondzadeh S, Mohammadi N, Noroozian M, Karamghadiri N, Ghoreishi A, Jamshidi AH, Forghani S (2009) Added ondansetron for stable schizophrenia: a double blind, placebo controlled trial. Schizophr Res 107:206–212
Akhondzadeh S, Ghayyoumi R, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, Naderi M, Ghebleh F, Tabrizi M, Rezazadeh SA (2011) Sildenafil adjunctive therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled trial. Psychopharmacology (Berl) 213:809–815
Arbabi M, Bagheri M, Rezaei F, Ahmadi-Abhari SA, Tabrizi M, Khalighi-Sigaroudi F, Akhondzadeh S (2012) A placebo-controlled study of the modafinil added to risperidone in chronic schizophrenia. Psychopharmacology (Berl) 220:591–598
Bennett AC, Vila TM (2010) The role of ondansetron in the treatment of schizophrenia. Ann Pharmacother 44:1301–1306
Briskin JK, Curtis JL (1997) Augmentation of clozapine therapy with ondansetron. Am J Psychiatry 154:1171
Buchanan RW (2007) Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 33:1013–1022
Chouinard G, Ross-Chouinard A, Annable L, Jones B (1980) Extrapyramidal symptom rating scale. Can J Neurol Sci 7:233
Costall B, Naylor RJ (2004) 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord 3:27–37
Den Boer JA, Vahlne JO, Post P, Heck AH, Daubenton F, Olbrich R (2000) Ritanserin as add-on medication to neuroleptic therapy for patients with chronic or subchronic schizophrenia. Hum Psychopharmacol 15:179–189
Duinkerke SJ, Botter PA, Jansen AA, Van Dongen PA, Van Haaften AJ, Boom AJ, Van Laarhoven JH, Busard HL (1993) Ritanserin, a selective 5-HT2/1C antagonist, and negative symptoms in schizophrenia. A placebo-controlled double-blind trial. Br J Psychiatry 163:451–455
Fenton WS, Mcglashan TH (1991) Natural history of schizophrenia subtypes. II. Positive and negative symptoms and long-term course. Arch Gen Psychiatry 48:978–986
Ghaleiha A, Noorbala AA, Farnaghi F, Hajiazim M, Akhondzadeh S (2010) A double-blind, randomized, and placebo-controlled trial of buspirone added to risperidone in patients with chronic schizophrenia. J Clin Psychopharmacol 30:678–682
Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, Mccarley R, Coyle JT (1999) A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 56:21–27
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hashimoto K, Iyo M, Freedman R, Stevens KE (2005) Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology (Berl) 183:13–19
Heresco-Levy U, Javitt DC, Ermilov M, Silipo G, Shimoni J (1998) Double-blind, placebo-controlled, crossover trial of d-cycloserine adjuvant therapy for treatment-resistant schizophrenia. Int J Neuropsychopharmacol 1:131–135
Kay SR, Fiszbein A, Opler LA (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32:214–219
Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, Nakazato M, Okamura N, Stevens KE, Freedman R, Iyo M (2005) Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res 76:67–72
Laughren T, Levin R (2006) Food and Drug Administration perspective on negative symptoms in schizophrenia as a target for a drug treatment claim. Schizophr Bull 32:220–222
Levkovitz Y, Arnest G, Mendlovic S, Treves I, Fennig S (2005) The effect of ondansetron on memory in schizophrenic patients. Brain Res Bull 65:291–295
Liang X, Arvanov VL, Wang RY (1998) Inhibition of NMDA-receptor mediated response in the rat medial prefrontal cortical pyramidal cells by the 5-HT3 receptor agonist SR 57227A and 5-HT: intracellular studies. Synapse 29:257–268
Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL (2000) Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry 157:767–771
Martin LF, Kem WR, Freedman R (2004) Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 174:54–64
Meltzer HY (1995) Role of serotonin in the action of atypical antipsychotic drugs. Clin Neurosci 3:64–75
Murphy BP, Chung YC, Park TW, Mcgorry PD (2006) Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res 88:5–25
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, Mclean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128:419–432
Potvin S, Stip E, Roy JY (2003) Clozapine, quetiapine and olanzapine among addicted schizophrenic patients: towards testable hypotheses. Int Clin Psychopharmacol 18:121–132
Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, Haraguchi T, Kanahara N, Shiraishi T, Fujisaki M, Fukami G, Nakazato M, Iyo M, Hashimoto K (2010) A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry 9:27
Sirota P, Mosheva T, Shabtay H, Giladi N, Korczyn AD (2000) Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry 157:287–289
White A, Corn TH, Feetham C, Faulconbridge C (1991) Ondansetron in treatment of schizophrenia. Lancet 337:1173
Wildeboer KM, Zheng L, Choo KS, Stevens KE (2009) Ondansetron results in improved auditory gating in DBA/2 mice through a cholinergic mechanism. Brain Res 1300:41–50
Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ (2006) Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr Res 88:102–110
Acknowledgments
This study was Dr. Sina Ghasemi’s postgraduate thesis toward the Iranian Board of Psychiatry. This study was supported by a grant from Tehran University of Medical Sciences to Prof. Shahin Akhondzadeh (grant no. 11426).
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Noroozian, M., Ghasemi, S., Hosseini, SMR. et al. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology 228, 595–602 (2013). https://doi.org/10.1007/s00213-013-3064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3064-2