Abstract
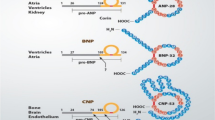
Since the discovery of atrial natriuretic factor by de Bold et al., there has been tremendous progress in our understanding of the physiologic, diagnostic and therapeutic roles of the natriuretic peptides (NPs) in health and disease. Natriuretic peptides are endogenous hormones that are released by the heart in response to myocardial stretch and overload. Three mammalian NPs have been identified and characterized, including atrial natriuretic peptide (ANP or atrial natriuretic factor), B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). In addition, Dendroaspis natriuretic peptide (DNP) has been isolated from the venom of Dendroaspis angusticeps (the green mamba snake), and urodilatin from human urine. These peptides are structurally similar and they consist of a 17-amino-acid core ring and a cysteine bridge. Both ANP and BNP bind to natriuretic peptide receptor A (NPR-A) that are expressed in the heart and other organs. Activation of NPR-A generates an increase in cyclic guanosine monophosphate, which mediates natriuresis, inhibition of renin and aldosterone, as well as vasorelaxant, anti-fibrotic, anti-hypertrophic, and lusitropic effects. The NP system thus serves as an important compensatory mechanism against neurohumoral activation in heart failure. This provides a strong rationale for the use of exogenous NPs in the management of acutely decompensated heart failure. In this article, the therapeutic applications of NPs in the acute heart failure syndromes are reviewed. Emerging therapeutic agents and areas for future research are discussed.



Similar content being viewed by others
References
de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H (1981) A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28:89–94
Boerrigter G, Burnett JC Jr (2004) Cardiorenal syndrome in decompensated heart failure: prognostic and therapeutic implications. Curr Heart Fail Rep 1:113–120
Chen HH, Burnett JC Jr (2006) Clinical application of the natriuretic peptides in heart failure. Eur Heart J 8(Suppl E):E18–E25
Potter LR, Abbey-Hosch S, Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27:47–72
Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M (1992) A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem 267:13928–13932
Schirger JA, Heublein DM, Chen HH, Lisy O, Jougasaki M, Wennberg PW et al (1999) Presence of Dendroaspis natriuretic peptide-like immunoreactivity in human plasma and its increase during human heart failure. Mayo Clin Proc 74:126–130
Margulies KB, Burnett JC Jr (2006) Visualizing the basis for paracrine natriuretic peptide signaling in human heart. Circ Res 99:113–115
Forssmann W, Meyer M, Forssmann K (2001) The renal urodilatin system: clinical implications. Cardiovasc Res 51:450–462
Burnett JC Jr, Costello-Boerrigter L, Boerrigter G (2004) Alterations in the kidney in heart failure: the cardiorenal axis in the regulation of sodium homeostasis. In: Mann DL (ed) Heart failure: a companion to Braunwald’s heart disease. Elsevier Inc, Philadelphia, pp 279–289
Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G et al (1992) Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130:229–239
Singh G, Kuc RE, Maguire JJ, Fidock M, Davenport AP (2006) Novel snake venom ligand Dendroaspis natriuretic peptide is selective for natriuretic peptide receptor-A in human heart: downregulation of natriuretic peptide receptor-A in heart failure. Circ Res 99:183–190
Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP et al (1986) Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 231:1145–1147
Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y et al (1991) Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 87:1402–1412
Sudoh T, Minamino N, Kangawa K, Matsuo H (1990) C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun 168:863–870
Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC Jr (1992) Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol 263:H1318–1321
Del Ry S, Passino C, Emdin M, Giannessi D (2006) C-type natriuretic peptide and heart failure. Pharmacol Res 54:326–333
Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V et al (2006) Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab 17:251–258
Ahluwalia A, MacAllister RJ, Hobbs AJ (2004) Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol 99:83–89
Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H et al (1991) Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252:120–123
Wei CM, Aarhus LL, Miller VM, Burnett JC Jr (1993) Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol 264:H71–H73
Wennberg PW, Miller VM, Rabelink T, Burnett JC Jr (1999) Further attenuation of endothelium-dependent relaxation imparted by natriuretic peptide receptor antagonism. Am J Physiol 277:H1618–H1621
Stingo AJ, Clavell AL, Aarhus LL, Burnett JC Jr (1992) Cardiovascular and renal actions of C-type natriuretic peptide. Am J Physiol 262:H308–H312
Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K et al (1991) C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem Biophys Res Commun 177:927–931
Cao L, Gardner DG (1995) Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25:227–234
Tokudome T, Horio T, Soeki T, Mori K, Kishimoto I, Suga S-i et al (2004) Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-1 signaling pathways. Endocrinology 145:2131–2140
Igaki T, Itoh H, Suga SI, Hama N, Ogawa Y, Komatsu Y et al (1998) Effects of intravenously administered C-type natriuretic peptide in humans: comparison with atrial natriuretic peptide. Hypertens Res 21:7–13
Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG (1994) Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab 78:1428–1435
Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A (2004) Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation 110:1231–1235
Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A et al (2005) C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci USA 102:14452–14457
Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K et al (2005) C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol 45:608–616
Nazario B, Hu RM, Pedram A, Prins B, Levin ER (1995) Atrial and brain natriuretic peptides stimulate the production and secretion of C-type natriuretic peptide from bovine aortic endothelial cells. J Clin Invest 95:1151–1157
Carstens J, Jensen KT, Pedersen EB (1998) Metabolism and action of urodilatin infusion in healthy volunteers. Clin Pharmacol Ther 64:73–86
Saxenhofer H, Fitzgibbon WR, Paul RV (1993) Urodilatin: binding properties and stimulation of cGMP generation in rat kidney cells. Am J Physiol 264:F267–273
Koike J, Nonoguchi H, Terada Y, Tomita K, Marumo F (1993) Effect of urodilatin on cGMP accumulation in the kidney. J Am Soc Nephrol 3:1705–1709
Goetz K, Drummer C, Zhu JL, Leadley R, Fiedler F, Gerzer R (1990) Evidence that urodilatin, rather than ANP, regulates renal sodium excretion. J Am Soc Nephrol 1:867–874
Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA et al (2005) Acute heart failure syndromes: Current state and framework for future research. Circulation 112:3958–3968
Burnett JC (2005) Nesiritide: new hope for acute heart failure syndromes? Eur Heart J 7(Suppl B):B25–B30
Tan AC, Russel FG, Thien T, Benraad TJ (1993) Atrial natriuretic peptide. An overview of clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 24:28–45
Nakao K, Sugawara A, Morii N, Sakamoto M, Yamada T, Itoh H et al (1986) The pharmacokinetics of alpha-human atrial natriuretic polypeptide in healthy subjects. Eur J Clin Pharmacol 31:101–103
Weidmann P, Hasler L, Gnadinger MP, Lang RE, Uehlinger DE, Shaw S et al (1986) Blood levels and renal effects of atrial natriuretic peptide in normal man. J Clin Invest 77:734–742
Eiskjaer H, Pedersen EB (1993) Dose-response study of atrial natriuretic peptide bolus injection in healthy man. Eur J Clin Invest 23:37–45
Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, Ryman KS et al (1986) Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest 78:1362–1374
Lindenfeld J, Schrier RW (2007) The kidney in heart failure. In: Hosenpud JD, Greenberg BH (eds) Congestive heart failure, 3rd edn.Lippincott Williams & Wilkins, Philadelphia, PA, pp 243–260
Hawkridge AM, Heublein DM, Bergen HR 3rd, Cataliotti A, Burnett JC Jr, Muddiman DC (2005) Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA 102:17442–17447
Giles TD, Quiroz AC, Roffidal LE, Marder H, Sander GE (1991) Prolonged hemodynamic benefits from a high-dose bolus injection of human atrial natriuretic factor in congestive heart failure. Clin Pharmacol Ther 50:557–563
Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K (2005) Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J Mar 69(3):283–90
Keating GM, Goa KL (2003) Nesiritide: a review of its use in acute decompensated heart failure. Drugs 63:47–70
Sackner-Bernstein JD, Skopicki H, Aaronson KD (2006) Natriuretic peptides for the treatment of heart failure. In: Feldman A (ed) Heart failure: pharmacologic management. Blackwell Publishing, Malden, MA, pp 154–171
Heart Failure Society of America. HFSA (2006) Comprehensive heart failure practice guideline: Section 12: Evaluation and management of patients with acute decompensated heart failure. J Card Fail 12:e86–e103
Emdin M, Clerico A (2006) Cardiac natriuretic hormone system as target for cardiovascular therapy. In: Clerico A, Emdin M (eds) Natriuretic peptides: the hormones of the heart. Springer-Verlag, New York, pp 161–175
Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD et al (2000) for the nesiritide study group. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure N Engl J Med 343:246–253
Publication Committee for the VMAC Investigators (Vasodilation in the Management of Acute CHF) (2002) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 287:1531–1540 [Erratum, JAMA (2002) 1288:1577]
Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 46:e1–e82
Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G et al (2005) Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the task force on acute heart failure of the European society of cardiology. Eur Heart J 26:384–416
Adams KF, Lindenfeld J, Arnold JMO, Baker DW, Barnard DH, Baughman KL et al HFSA (2006) Comprehensive Heart Failure Practice Guidelines. J Cardiac Failure 12:e1–e122
Dorner GT, Selenko N, Kral T, Schmetterer L, Eichler HG, Wolzt M (1998) Hemodynamic effects of continuous urodilatin infusion: a dose-finding study. Clin Pharmacol Ther 64:322–330
Bestle MH, Olsen NV, Christensen P, Jensen BV, Bie P (1999) Cardiovascular, endocrine, and renal effects of urodilatin in normal humans. Am J Physiol 276:R684–R695
Saxenhofer H, Raselli A, Weidmann P, Forssmann WG, Bub A, Ferrari P et al (1990) Urodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in men. Am J Physiol 259:F832–F838
Mitrovic V, Luss H, Nitsche K, Forssmann K, Maronde E, Fricke K et al (2005) Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J 150:1239
Cleland JGF, Coletta AP, Lammiman M, Witte KK, Loh H, Nasir M et al (2005) Clinical trials update from the European Society of Cardiology meeting 2005: CARE-HF extension study, ESSENTIAL, CIBIS-III, S-ICD, ISSUE-2, STRIDE-2, SOFA, IMAGINE, PREAMI, SIRIUS-II and ACTIVE. Eur J Heart Fail 7:1070–1075
Chen HH, Schirger JA, Alessandro C, Martin FL, Burnett JC (2006) Intra-renal infusion of BNP in experimental heart failure: A novel strategy to maximize the renal enhancing actions of BNP while minimizing arterial hypotension [abst]. J Card Fail 12:S32
Mathur VS, Goodson B, Patel S, Valencia A, Elkins J (2004) Evidence for substantial renal first pass effects of human b-type natriuretic peptide (nesiritide) following intra-renal infusion [abst]. J Card Fail 10:S68
Heywood JT, Ho A, Mathur V (2006) Favorable renal hemodynamic effects of intra-renal nesiritide infusion in heart transplant patients [abst]. J Card Fail 12:S3
Riter HG, Redfield MM, Burnett JC, Chen HH (2006) Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction [letter]. J Am Coll Cardiol 47:2334–2335
Luber JM Jr, The NAPA Investigators (2006) Perioperative nesiritide use is associated with decreased 180-day mortality in heart failure patients undergoing cardiothoracic surgery [abst]. J Card Fail 12:S73–S74
Oz MC. on behalf of the NAPA Investigators. Effect of perioperative nesiritide administration on postoperative renal function and clinical outcomes in patients undergoing cardiothoracic surgery: Results of the NAPA trial [abst]. Available at http://cicescardioorg/AbstractDetailsaspx?id = 28005 Accessed September 2, 2006
Zierer A, Safarzadeh E, Ewald GA, Pasque MK, Moon MR, Lawton JS et al (2006) Potential renal protective benefits of intra-operative BNP infusion during cardiac transplantation [abst]. Transplantation 82:564
Burnett JC Jr, Opgenorth TJ, Granger JP (1986) The renal action of atrial natriuretic peptide during control of glomerular filtration. Kidney Int 30:16–19
Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD (2001) Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol 37:1221–1227
Bestle MH, Bie P (1993) Renal effects of urodilatin and atrial natriuretic peptide in volume expanded conscious dogs. Acta Physiol Scand 149:77–83
Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC Jr (2000) Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol 36:1706–1712
Chen HH, Lainchbury JG, Harty GJ, Burnett JC Jr (2002) Maximizing the natriuretic peptide system in experimental heart failure: subcutaneous brain natriuretic peptide and acute vasopeptidase inhibition. Circulation 105:999–1003
Chen HH, Schirger JA, Cataliotti A, Burnett JC Jr (2006) Intact acute cardiorenal and humoral responsiveness following chronic subcutaneous administration of the cardiac peptide BNP in experimental heart failure. Eur J Heart Fail published online Feb 2, 2006
Chen HH, Huntley BK, Schirger JA, Cataliotti A, Burnett JC Jr (2006) Maximizing the renal cyclic 3′-5′-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: A novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol 17:2742–2747
Chen HH, Redfield MM, Nordstrom LJ, Horton DP, Burnett JC Jr (2004) Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail 10:115–119
Madhani M, Okorie M, Hobbs AJ, Macallister RJ (2006) Reciprocal regulation of human soluble and particulate guanylate cyclases in vivo. Br J Pharmacol published online October 3, 2006
Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP (2006) NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5:755–768
Banga AK (2006) Oral delivery of peptide and protein drugs. Therapeutic peptides and proteins: formulation, processing, and delivery systems, 2nd edn. CRC Press, Boca Raton, FL, pp 229–258
Cataliotti A, Schirger JA, Martin FL, Chen HH, McKie PM, Boerrigter G et al (2005) Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation 112:836–840
Cataliotti A, Heublein DM, James KD, Burnett JC (2006) Biological actions of a novel oral human BNP in an experimental model of acute hypertension [abst]. J Card Fail 12:S30
Wang W, Ou Y, Shi Y (2004) AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm Res 21:2105–2111
Aaranson KD, Sackner-Bernstein JD (2006) Risk of death associated with nesiritide in patients with acutely decompensated heart failure. JAMA 296:1465–1466
Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K (2005) Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 293:1900–1905
Sackner-Bernstein JD, Skopicki HA, Aaronson KD (2005) Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111:1487–1491
Braunwald E, Burnett JC Jr, Colucci WS, et al. Natrecor Advisory Panel Report. In: Panel of cardiology experts provides recommendations to Scios regarding Natrecor. Healthcare Professional Letter. Fremont, CA: Scios June 13, 2005. Available at www.natrecor.com/pdf/Braunwald_Panel_Release_&_Report.pdf Accessed September 12, (2006)
Panel of cardiology experts provides recommendations to Scios regarding Natrecor. Healthcare Professional Letter. Fremont, CA: Scios June 13, 2005. Available at www.natrecor.com/pdf/Braunwald_Panel_Release_&_Report.pdf Accessed September 12, (2006)
Biollaz J, Nussberger J, Porchet M, Brunner-Ferber F, Otterbein ES, Gomez H et al (1986) Four-hour infusions of synthetic atrial natriuretic peptide in normal volunteers. Hypertension 8:II96–II105
Kentsch M, Drummer C, Gerzer R, Muller-Esch G (1995) Severe hypotension and bradycardia after continuous intravenous infusion of urodilatin (ANP 95-126) in a patient with congestive heart failure. Eur J Clin Invest 25:281–283
Burnett J, Boerrigter G (2005) cGMP enhancing strategies for acute and chronic heart failure [abst]. BMC Pharmacology 5:S22
Grantham JA, Borgeson DD, Burnett JC Jr (1997) BNP: pathophysiological and potential therapeutic roles in acute congestive heart failure. Am J Physiol 272:R1077–R1083
Best PJ, Burnett JC, Wilson SH, Holmes DR Jr, Lerman A (2002) Dendroaspis natriuretic peptide relaxes isolated human arteries and veins. Cardiovasc Res 55:375–384
Collins E, Bracamonte MP, Burnett JC Jr, Miller VM (2000) Mechanism of relaxations to dendroaspis natriuretic peptide in canine coronary arteries. J Cardiovasc Pharmacol 35:614–618
Singh G, Maguire JJ, Kuc RE, Skepper JN, Fidock M, Davenport AP (2006) Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br J Pharmacol published online October 16, 2006
Lainchbury JG, Lisy O, Burnett JC Jr, Meyer DM, Redfield MM (2002) Actions of a novel synthetic natriuretic peptide on hemodynamics and ventricular function in the dog. Am J Physiol Regul Integr Comp Physiol 282:R993–R998
Lisy O, Jougasaki M, Heublein DM, Schirger JA, Chen HH, Wennberg PW et al (1999) Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int 56:502–508
Lisy O, Lainchbury JG, Leskinen H, Burnett JC Jr (2001) Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension 37:1089–1094
Chen HH, Lainchbury JG, Burnett JC Jr (2002) Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J Am Coll Cardiol 40:1186–1191
Lewis RJ, Garcia ML (2003) Therapeutic potential of venom peptides. Nat Rev Drug Discov 2:790–802
Fry BG (2005) From genome to “venome”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res 15:403–420
Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF et al (2006) Early evolution of the venom system in lizards and snakes. Nature 439:584–588
Bazaa A, Marrakchi N, El Ayeb M, Sanz L, Calvete JJ (2005) Snake venomics: comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics 5:4223–4235
Barbouche R, Marrakchi N, Mansuelle P, Krifi M, Fenouillet E, Rochat H et al (1996) Novel anti-platelet aggregation polypeptides from Vipera lebetina venom: Isolation and characterization. FEBS Lett 392:6–10
Amininasab M, Elmi MM, Endlich N, Endlich K, Parekh N, Naderi-Manesh H et al (2004) Functional and structural characterization of a novel member of the natriuretic family of peptides from the venom of Pseudocerastes persicus. FEBS Lett 557:104–108
Michel GH, Murayama N, Sada T, Nozaki M, Saguchi K, Ohi H et al (2000) Two N-terminally truncated forms of C-type natriuretic peptide from habu snake venom. Peptides 21:609–615
Fry BG, Wickramaratana JC, Lemme S, Beuve A, Garbers D, Hodgson WC et al (2005) Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): isolation, chemical and biological characterisation. Biochem Biophys Res Commun 327:1011–1015
Ho PL, Soares MB, Maack T, Gimenez I, Puorto G, Furtado MF et al (1997) Cloning of an unusual natriuretic peptide from the South American coral snake Micrurus corallinus. Eur J Biochem 250:144–149
St Pierre L, Woods R, Earl S, Masci PP, Lavin MF (2005) Identification and analysis of venom gland-specific genes from the coastal taipan (Oxyuranus scutellatus) and related species. Cell Mol Life Sci 62:2679–2693
St Pierre L, Flight S, Masci PP, Hanchard KJ, Lewis RJ, Alewood PF et al (2006) Cloning and characterisation of natriuretic peptides from the venom glands of Australian elapids. Biochimie published online August 4, 2006
Murayama N, Hayashi MA, Ohi H, Ferreira LA, Hermann VV, Saito H et al (1997) Cloning and sequence analysis of a Bothrops jararaca cDNA encoding a precursor of seven bradykinin-potentiating peptides and a C-type natriuretic peptide. Proc Natl Acad Sci USA 94:1189–1193
Soares MR, Oliveira-Carvalho AL, Wermelinger LS, Zingali RB, Ho PL, Junqueira-de-Azevedo Ide L et al (2005) Identification of novel bradykinin-potentiating peptides and C-type natriuretic peptide from Lachesis muta venom. Toxicon 46:31–38
Ianzer D, Konno K, Marques-Porto R, Vieira Portaro FC, Stocklin R, Martins de Camargo AC et al (2004) Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides 25:1085–1092
Hayashi MA, Camargo AC (2005) The Bradykinin-potentiating peptides from venom gland and brain of Bothrops jararaca contain highly site specific inhibitors of the somatic angiotensin-converting enzyme. Toxicon 45:1163–1170
Wei CM, Kim CH, Miller VM, Burnett JC Jr (1993) Vasonatrin peptide: a unique synthetic natriuretic and vasorelaxing peptide. J Clin Invest 92:2048–2052
Lisy O, Burnett JC Jr (2003) The design, synthesis and cardiorenal actions of a new chimeric natriuretic peptide CD-NP [abst]. J Am Coll Cardiol 41:312A
Lisy O, Burnett JC (2003) The new designer peptide CD-NP unloads the heart, suppresses renin and is natriuretic in vivo [abst]. J Card Fail 9:S32
Cataliotti A, Boerrigter G, Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM et al (2004) Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation 109:1680–1685
Boerrigter G, Costello-Boerrigter LC, Lapp H, Stasch J-P, Burnett JC Jr (2005) Co-activation of soluble and particulate guanylate cyclase by BAY 58–2667 and BNP enhances cardiorenal function in experimental heart failure [abst]. J Card Fail 11(Suppl 1):S89
Costello-Boerrigter LC, Boerrigter G, Harty GJ, Burnett JC Jr (2006) Co-targeting of the V2 receptor with tolvaptan and the natriuretic peptide A-receptor with B-type natriuretic peptide enhances water and sodium excretion without adversely affecting renal function: A novel physiologic approach to sodium and water retention in experimental heart failure [abst]. J Card Fail 12:S84–S85
Hobbs AJ (2002) Soluble guanylate cyclase: An old therapeutic target re-visited. Br J Pharmacol 136:637–640
Friebe A, Koesling D (2003) Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res 93:96–105
Rothkegel C, Schmidt PM, Stoll F, Schroder H, Schmidt HH, Stasch JP (2006) Identification of residues crucially involved in soluble guanylate cyclase activation. FEBS Lett 580:4205–4213
Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M et al (2002) NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136:773–783
Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM (1994) YC-1, a novel activator of platelet guanylate cyclase. Blood 84:4226–4233
Wu CC, Ko FN, Kuo SC, Lee FY, Teng CM (1995) YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br J Pharmacol 116:1973–1978
Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A et al (2001) NO-independent regulatory site on soluble guanylate cyclase. Nature 410:212–215
Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H et al (2003) Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41-2272 in experimental congestive heart failure. Circulation 107:686–689
Stasch J-P, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, AK HS, Meurer S et al (2006) Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116:2552–2561
Scios announces the ETNA clinical trial with nesiritide: ETNA trial to be largest study of acute heart failure ever conducted. Press release September 21, 2005. Available at http://www.sciosinc.com/scios/pr_1127326387 Accessed September 29, 2006
Stiles S. Nesiritide shows hint of survival benefit in chronic-HF patients undergoing CABG. theheart.org. [HeartWire>Heart failure] September 12, 2006. Available at http://www.theheart.org/article/741265.do Accessed Sep 12, 2006
Baxter GF (2004) Natriuretic peptides and myocardial ischaemia. Basic Res Cardiol 99:90–93
Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V-P, et al (2006) EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J published online September 25, 2006:ehl193
Sezai A, Shiono M, Orime Y, Hata H, Hata M, Negishi N et al (2000) Low-dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Ann Thorac Surg 69:732–738
Wajima Z, Shiga T, Imanaga K, Inoue T, Ogawa R (2006) Effect of prophylactic bronchodilator treatment with i.v. carperitide on airway resistance and lung compliance after tracheal intubation. Br J Anaesth 96:660–664
Acknowledgments
Supported by grants from the National Institutes of Health (HL36634; PO1 HL76611 and HL80732) and the Mayo Foundation. Dr. Lee is supported by the Clinical Research Initiative Fellowship from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, C.Y.W., Burnett, J.C. Natriuretic peptides and therapeutic applications. Heart Fail Rev 12, 131–142 (2007). https://doi.org/10.1007/s10741-007-9016-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-007-9016-3