Abstract
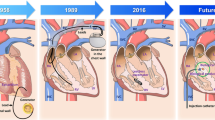
The prevention and treatment of cardiac arrhythmias conferring major morbidity and mortality is far from optimal, and relies heavily on devices and drugs for the partial successes that have been seen. The greatest success has been in the use of electronic pacemakers to drive the hearts of patients having high degree heart block. Recent years have seen the beginnings of attempts to use novel approaches available through gene and cell therapies to treat both brady- and tachyarrhythmias. By far the most successful approaches to date have been seen in the development of biological pacemakers. However, the far more difficult problems posed by atrial fibrillation and ventricular tachycardia are now being addressed. In the following pages we review the approaches now in progress as well as the specific methodologic demands that must be met if these therapies are to be successful.





Similar content being viewed by others
References
Huikuri, H. V., Castellanos, A., & Myerburg, R. J. (2001). Sudden death due to cardiac arrhythmias. New England Journal of Medicine, 345, 1473–1482 Medline DOI 10.1056/NEJMra000650.
Huikuri, H. V., Makikallio, T. H., Raatikainen, M. J., Perkiomaki, J., Castellanos, A., & Myerburg, R. J. (2003). Prediction of sudden cardiac death: Appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation, 108, 110–115 Medline. DOI 10.1161/01.CIR.0000077519.18416.43.
Zipes, D. P., & Wellens, H. J. J. (1998). Sudden cardiac death. Circulation, 98, 2334–2351 Medline.
Myerburg, R. J., Feigal, D. W. Jr, & Lindsay, B. D. (2006). Life-threatening malfunction of implantable cardiac devices. New England Journal of Medicine, 354, 2309–2311 Medline. DOI 10.1056/NEJMp068112.
Go, A. S., Hylek, E. M., Phillips, K. A., et al. (2001). Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA, 285, 2370–2375 Medline. DOI 10.1001/jama.285.18.2370.
Miyasaka, Y., Barnes, M. E., Gersh, B. J., et al. (2006). Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation, 114, 119–125 Medline. DOI 10.1161/CIRCULATIONAHA.105.595140.
Fuster, V., Ryden, L. E., Cannom, D. S., et al. (2006). American College of Cardiology. American Heart Association Task Force on Practice Guidelines. European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. Journal of the American College of Cardiology, 48, 854–906 Medline. DOI 10.1016/j.jacc.2006.07.009.
Withering, W. (1979). An account of the foxglove and some of its medical uses (1785). Birmingham: Classics of Medicine Library.
Scherf, D., & Schott, A. (1973). Extrasystoles and allied arrhythmias (2nd ed.). Great Britain: Year Book Medical Publishers.
Harris, A. S., & Kokernot, R. H. (1950). Effects of diphenylhydantoin sodium (dilantin sodium) and phenobarbital sodium upon ectopic ventricular tachycardia in acute myocardial infarction. American Journal of Physiology, 163, 505–516 Medline.
Vaughan Williams, E. M. (1979). Classification of antiarrhythmic drugs. In E. Sandoe, E. Flensted-Jensen, & K. H. Olesen (Eds.) Symposium on cardiac arrhythmias (pp. 449–472). Astra: Sodertalje.
Echt, D. S., Liebson, P. R., Mitchell, L. B., et al. (1991). Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. New England Journal of Medicine, 324, 781–788 Medline.
Waldo, A. L., Camm, A. J., deRuyter, H., et al. (1996). Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet, 348, 7–12 Medline. DOI 10.1016/S0140-6736(96)02149-6.
Spooner, P. M., & Rosen, M. R. (2000). Perspectives on arrhythmogenesis, antiarrhythmic strategies and sudden cardiac death. In P. M. Spooner, & M. R. Rosen (Eds.) Foundations of cardiac arrhythmias (pp. 1–20). New York: Marcel Dekker.
Vermes, E., Tardif, J. C., Bourassa, M. G., et al. (2003). Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: Insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation, 107, 2926–2931 Medline. DOI 10.1161/01.CIR.0000072793.81076.D4.
Ducharme, A., Swedberg, K., Pfeffer, M. A., et al. (2006). CHARM Investigators. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. American Heart Journal, 152, 86–92 Medline. DOI 10.1016/j.ahj.2005.06.036.
Crijns, H. J., Van den Berg, M. P., Van Gelder, I. C., & Van Veldhuisen, D. J. (1997). Management of atrial fibrillation in the setting of heart failure. European Heart Journal, 18, C45–C49 Medline.
Pedersen, O. D., Henning, B., Køber, L., & Torp-Pedersen, C. (1999). Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation, 100, 376–380 Medline.
Moss, A. J., Hall, W. J., Cannom, D. S., et al. (1996). MADIT Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. New England Journal of Medicine, 335, 1933–1940 Medline. DOI 10.1056/NEJM199612263352601.
Moss, A. J., Zareba, W., Hall, W. J., Klein, H., Wilber, D. J., Cannom, D. S., et al. (2002). Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New England Journal of Medicine, 346, 877–883 Medline. DOI 10.1056/NEJMoa013474.
Bardy, G. H., Lee, K. L., Mark, D. B., et al. (2005). Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. New England Journal of Medicine, 352, 225–237 Medline. DOI 10.1056/NEJMoa043399.
Kadish, A., Dyer, A., Daubert, J. P., et al. (2004). Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. New England Journal of Medicine, 350, 2151–2158 Medline. DOI 10.1056/NEJMoa033088.
Bristow, M. R., Saxon, L. A., Boehmer, J., et al. (2004). Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. New England Journal of Medicine, 350, 2140–2150 Medline. DOI 10.1056/NEJMoa032423.
Adamson, P. B., Barr, R. C., Callan, D. J., et al. (2005). The perplexing complexity of cardiac arrhythmias: Beyond electrical remodeling. Heart Rhythm, 2, 650–659 Medline. DOI 10.1016/j.hrthm.2005.03.009.
Members of the Sicilian Gambit (2001). New approaches to antiarrhythmic therapy. Emerging therapeutic applications of the cell biology of cardiac arrhythmias. European Heart Journal, 22, 2148–2163 Medline. DOI 10.1053/euhj.2001.3036.
Rosen, M. (2005). Biological pacemaking: In our lifetime? Heart Rhythm, 2, 418–428 Medline. DOI 10.1016/j.hrthm.2004.12.016.
Cohen, I. S., Brink, P. R., Robinson, R. B., & Rosen, M. R. (2005). The why, what, how and when of biological pacemakers. Nature Clinical Practice Cardiovascular Medicine, 2, 374–375 Medline. DOI 10.1038/ncpcardio0276.
Edelberg, J. M., Huang, D. T., Josephson, M. E., & Rosenberg, R. D. (2001). Molecular enhancement of porcine cardiac chronotropy. Heart, 86, 559–562 Medline. DOI 10.1136/heart.86.5.559.
Miake, J., Marban, E., & Nuss, H. B. (2002). Gene therapy: Biological pacemaker created by gene transfer. Nature, 419, 132–133 Medline. DOI 10.1038/419132b.
Qu, J., Plotnikov, A. N., Danilo Jr, P., et al. (2003). Expression and function of a biological pacemaker in canine heart. Circulation, 107, 1106–1109 Medline. DOI 10.1161/01.CIR.0000059939.97249.2C.
Potapova, I., Plotnikov, A., Lu, Z., et al. (2004). Human mesenchymal stem cell as a gene delivery system to create cardiac pacemakers. Circulation Research, 94, 841–959 DOI 10.1161/01.RES.0000123827.60210.72.
Bucchi, A., Plotnikov, A. N., Shlapakova, I., Danilo, P. Jr., Kryukova, Y., Qu, J., et al. (2006). Wild-type and mutant HCN channels in a tandem biological–electronic cardiac pacemaker. Circulation, 114, 992–999 Medline. DOI 10.1161/CIRCULATIONAHA.106.617613.
Tse, H. F., Xue, T., Lau, C. P., et al. (2006). Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN Channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation, 114, 1000–1011 Medline. DOI 10.1161/CIRCULATIONAHA.106.615385.
Kashiwakura, Y., Cho, H. C., Barth, A. S., Azene, E., & Marban, E. (2006). Gene transfer of a synthetic pacemaker channel into the heart: a novel strategy for biological pacing. Circulation, 114, 1682–1686 Medline. DOI 10.1161/CIRCULATIONAHA.106.634865.
Kehat, I., Khimovich, L., Caspi, O., et al. (2004). Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nature Biotechnology, 22, 1282–1289 Medline. DOI 10.1038/nbt1014.
Plotnikov, A. P., Shlapakova, I., Szabolcs, M. J., et al. (2007). Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation, 116, 706–713 Medline. DOI 10.1161/CIRCULATIONAHA.107.703231.
Sasano, T., McDonald, A. D., Kikuchi, K., & Donahue, J. K. (2006). Molecular ablation of ventricular tachycardia after myocardial infarction. Nature Medicine, 12, 1256–1258 Medline. DOI 10.1038/nm1503.
Kikuchi, K., McDonald, A. D., Sasano, T., & Donahue, J. K. (2005). Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation, 111, 264–270 Medline. DOI 10.1161/01.CIR.0000153338.47507.83.
Bauer, A., McDonald, A. D., Nasir, K., et al. (2004). Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation, 110, 3115–3120 Medline. DOI 10.1161/01.CIR.0000147185.31974.BE.
Murata, M., Cingolani, E., McDonald, A. D., Donahue, J. K., & Marban, E. (2004). Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circulation Research, 95, 398–405 Medline. DOI 10.1161/01.RES.0000138449.85324.c5.
Bunch, T. J., Mahapatra, S., Bruce, G. K., et al. (2006). Impact of transforming growth factor-beta1 on atrioventricular node conduction modification by injected autologous fibroblasts in the canine heart. Circulation, 113, 2485–2494 Medline. DOI 10.1161/CIRCULATIONAHA.105.570796.
Biel, M., Schneider, A., & Wahl, C. (2002). Cardiac HCN channels: structure, function, and modulation. Trends in Cardiovascular Medicine, 12, 206–212 Medline. DOI 10.1016/S1050-1738(02)00162-7.
Plotnikov, A. N., Sosunov, E. A., Qu, J., et al. (2004). Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation, 109, 506–512 Medline. DOI 10.1161/01.CIR.0000114527.10764.CC.
Choi, Y. H., Stamm, C., Hammer, P. E., et al. (2006). Cardiac conduction through engineered tissue. American Journal of Pathology, 169, 72–85 Medline. DOI 10.2353/ajpath.2006.051163.
Marban, E., Nuss, H. B., & Donahue, J. K. (2002). Gene therapy for cardiac arrhythmias. Cold Spring Harbor Symposia on Quantitative Biology, 67, 527–531 Medline. DOI 10.1101/sqb.2002.67.527.
Burton, D. Y., Song, C., Fishbein, I., et al. (2003). The incorporation of an ion channel gene mutation associated with the long QT syndrome (Q9E-hMiRP1) in a plasmid vector for site-specific arrhythmia gene therapy: in vitro and in vivo feasibility studies. Human Gene Therapy, 14, 907–922 Medline. DOI 10.1089/104303403765701196.
Perlstein, I., Burton, D. Y., Ryan, K., et al. (2005). Posttranslational control of a cardiac ion channel transgene in vivo: clarithromycin–hMiRP1–Q9E interactions. Human Gene Therapy, 16, 906–910 Medline DOI 10.1089/hum.2005.16.906.
Akar, F. G., Wu, R. C., Juang, G. J., et al. (2005). Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 288, H2887–H2896 Medline. DOI 10.1152/ajpheart.00320.2004.
Nuss, H. B., Johns, D. C., Kaab, S., et al. (1996). Reversal of potassium channel deficiency in cells from failing hearts by adenoviral gene transfer: A prototype for gene therapy for disorders of cardiac excitability and contractility. Gene Therapy, 3, 900–912 Medline.
Ennis, I. L., Li, R. A., Murphy, A. M., Marban, E., & Nuss, H. B. (2002). Dual gene therapy with SERCA1 and Kir2.1 abbreviates excitation without suppressing contractility. Journal of Clinical Investigation, 109, 393–400 Medline. DOI 10.1172/JCI200213359.
Donahue, J. K., Kikuchi, K., & Sasano, T. (2005). Gene therapy for cardiac arrhythmias. Trends in Cardiovascular Medicine, 15, 219–224 Medline. DOI 10.1016/j.tcm.2005.06.007.
Neyroud, N., Nuss, H. B., Leppo, M. K., Marban, E., & Donahue, J. K. (2002). Gene delivery to cardiac muscle. Methods in Enzymology, 346, 323–334 Medline. DOI 10.1016/S0076-6879(02)46064-8.
Lehnart, S. E., & Donahue, J. K. (2003). Coronary perfusion cocktails for in vivo gene transfer. Methods in Molecular Biology, 219, 213–218 Medline.
Roth, D. M., Lai, N. C., Gao, M. H., et al. (2004). Indirect intracoronary delivery of adenovirus encoding adenylyl cyclase increases left ventricular contractile function in mice. American Journal of Physiology. Heart and Circulatory Physiology, 287, H172–H177 Medline. DOI 10.1152/ajpheart.01009.2003.
Tomaselli, G. F., & Donahue, J. K. (2003). Somatic gene transfer and cardiac arrhythmias: Problems and prospects. Journal of Cardiovascular Electrophysiology, 14, 547–550 Medline. DOI 10.1046/j.1540-8167.2003.t01-1-02567.x.
Kornowski, R., Fuchs, S., Leon, M. B., & Epstein, S. E. (2000). Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation, 101, 454–458 Medline.
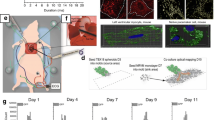
Rosen, M. R. (2006). Are stem cells drugs? Circulation, 114, 1992–2000 Medline. DOI 10.1161/CIRCULATIONAHA.106.641670.
Zimmett, J. M., & Hare, J. M. (2005). Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Research in Cardiology, 100, 471–481 Medline. DOI 10.1007/s00395-005-0553-4.
Sekar, R. B., Kizana, E., Smith, R. R., Barth, A. S., Zhang, Y., & Marban, E. (2007). Lentiviral vector-mediated expression of GFP or Kir2.1 alters the electrophysiology of neonatal rat ventricular myocytes without inducing cytotoxicity. American Journal of Physiology. Heart and Circulatory Physiology, 293, H2757–H2770.
Jackson, K. A., Majka, S. M., Wang, H., Pocius, J., Hartley, C. J., Majesky, M. W., et al. (2001). Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. Journal of Clinical Investigation, 107(11), 1395–1402.
Kajstura, J., Rota, M., Whang, B., Cascapera, S., Hosoda, T., Bearzi, C., et al. (2005). Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circulation Research, 96(1), 127–37.
Kraitchman, D. L., Heldman, A. W., Atalar, E., Amado, L. C., Martin, B. J., Pittenger, M. F., et al. (2003). In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation, 107(18), 2290–2293.
Dick, A. J., Guttman, M. A., Raman, V. K., Peters, D. C., Pessanha, B. S., Hill, J. M., et al. (2003). Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation, 108(23), 2899–2904.
Kraitchman, D. L., Tatsumi, M., Gilson, W. D., Ishimori, T., Kedziorek, D., Walczak, P., et al. (2005). Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation, 112(10), 1451–1461.
Barbash, I. M., Chouraqui, P., Baron, J., Feinberg, M. S., Etzion, S., Tessone, A., et al. (2003). Systemic delivery of bone marrow-derived mesenchymal stem cells to the infracted myocardium: feasibility, cell migration, and body distribution. Circulation, 108(7), 863–868.
Hofmann, M., Wollert, K. C., Meyer, G. P., Menke, A., Arseniev, L., Hertenstein, B., et al. (2005). Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation, 111(17), 2198–2202.
Laflamme, M. A., & Murry, C. E. (2005). Regenerating the heart. Nature Biotechnology, 23(7), 845–856.
Billinton, N., & Knight, A. W. (2001). Seeing the wood through the trees: a review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Analytical Biochemistry, 291(2), 175–197.
Rosen, A. B., Kelly, D. J., Schuldt, A. J. T., Lu, J., Potapova, I. A., Doronin, S. V., et al. (2007). Finding fluorescent needles in the cardiac haystack: Tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo 3-D fluorescence analysis. Stem Cells, 25, 2128–2138.
Ballou, B., Lagerholm, B. C., Eernst, L. A., Bruchez, M. P., & Waggoner, A. S. (2004). Noninvasive imaging of quantum dots in mice. Bioconjugate Chemistry, 75, 79–86.
Valiunas, V., Doronin, S., Valiuniene, L., Potapova, I., Zuckerman, J., Walcott, B., et al. (2004). Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. Journal of Physiology, 555, 617–626.
Valiunas, V., Polosina, Y. Y., Miller, H., Potapova, I. A., Valiuniene, L., Doronin, S., et al. (2005). Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. Journal of Physiology, 568, 459–468.
Plotnikov, A. N., Bucchi, A., Shlapakova, I. N., Danilo, P. Jr., Cohen, I. S., Brink, P. R., et al. (2006). Runaway biological pacemaker function induced by HCN212 is controlled by If blockade with ivabradine. Circulation, 114, II–123.
Plotnikov, A. P., Shlapakova, I., Szabolcs, M. J., Danilo, P. Jr., Lorell, B., Potapova, I. A., et al. (2007). Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation, 116, 706–713.
Potapova, I., Plotnikov, A., Lu, Z., Danilo, P. Jr., Valiunas, V., Qu, J., et al. (2004). Human mesenchymal stem cell as a gene delivery system to create cardiac pacemakers. Circulation Research, 94, 841–959.
Reinhard, E., Nedivi, E., Wegner, J., Skene, J. H. P., & Westerfield, M. (1994). Neural selective activation and temporal regulation of a mammalian GAP-43 promoter in zebrafish. Development, 120, 1767–1775.
Acknowledgments
The studies referred to were supported by USPHS NHLBI grants HL-28958 and HL-67101 and by Boston Scientific.
Conflict of Interest
The authors receive research support from Boston Scientific.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosen, M.R., Brink, P.R., Cohen, I.S. et al. Regenerative therapies in electrophysiology and pacing. J Interv Card Electrophysiol 22, 87–98 (2008). https://doi.org/10.1007/s10840-008-9208-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-008-9208-3