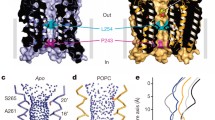
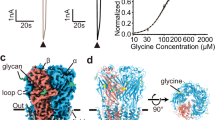
Abstract
The Cys-loop receptor family of ligand-gated ion channels (LGICs) play a key role in synaptic transmission in the central nervous system of animals. Recent advances have led to the elucidation of two crystal structures of related prokaryotic LGICs and the electron micrograph derived structure of the acetylcholine receptor from Torpedo marmorata. Here, we review the structural and biochemical data that form our understanding of the structure of the channel pore. We introduce original data from the glycine receptor using the substituted-cysteine accessibility technique and show that while the helical structure of the segment that surrounds the channel pore is generally agreed, the location of the channel gate, the pore diameter and the structure that forms the entry to the channel pore are likely to differ between receptors. The fundamental structural differences between anion and cation selective receptors and how these differences are related to the pore structure are also considered.




Similar content being viewed by others
References
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol 346:967–989. doi:10.1016/j.jmb.2004.12.031
Brejc K, van Dijk WJ, Klaassen RV et al (2001) Crystal structure of an ACh-binding protein that reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276. doi:10.1038/35077011
Celie PHN, Klaassen RV, van Rossum-Fikkert SE et al (2005) Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J Biol Chem 280:26457–26466. doi:10.1074/jbc.M414476200
Hansen SB, Sulzenbacher G, Huxford T et al (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24:3635–3646. doi:10.1038/sj.emboj.7600828
Lester HA, Dibas MI, Dahan DS et al (2004) Cys-loop receptors: new twists and turns. Trends Neurosci 27:329–336. doi:10.1016/j.tins.2004.04.002
Tasneem A, Iyer L, Jakobsson E et al (2004) Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6:R4. doi:10.1186/gb-2004-6-1-r4
Miyazawa A, Fujiyoshi Y, Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955. doi:10.1038/nature01748
Hilf RJC, Dutzler R (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452:375–379. doi:10.1038/nature06717
Hilf RJC, Dutzler R (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457:115–118. doi:10.1038/nature07461
Bocquet N, Nury H, Baaden M et al (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114. doi:10.1038/nature07462
Miller C (1989) Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron 2:1195–1205. doi:10.1016/0896-6273(89)90304-8
Dwyer TM, Adams DJ, Hille B (1980) The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol 75:469–492. doi:10.1085/jgp.75.5.469
Wang F, Imoto K (1992) Pore size and negative charge as structural determinants of permeability in the Torpedo nicotinic acetylcholine receptor channel. Proc R Soc Lond B Biol Sci 250:11–17. doi:10.1098/rspb.1992.0124
Cohen BN, Labarca C, Davidson N et al (1992) Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J Gen Physiol 100:373–400. doi:10.1085/jgp.100.3.373
Bocquet N, Prado de Carvalho L, Cartaud J et al (2007) A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 445:116–119. doi:10.1038/nature05371
Bormann J, Hamill OP, Sakmann B (1987) Mechanism of anion permeation through channels gated by glycine and g-aminobutyric acid in mouse cultured spinal neurons. J Physiol 385:243–286
Fatimashad K, Barry PH (1993) Anion permeation in GABA-Gated and glycine-gated channels of mammalian cultured hippocampal neurons. Proc R Soc Lond B Biol Sci 253:69–75. doi:10.1098/rspb.1993.0083
Rundström N, Schmeiden V, Betz H et al (1994) Cyanotriphenylborate: subtype-specific blocker of glycine receptor chloride channels. Proc Natl Acad Sci USA 91:8950–8954. doi:10.1073/pnas.91.19.8950
Wotring VE, Chang Y, Weiss DS (1999) Permeability and single channel conductance of human homomeric rho1 GABAC receptors. J Physiol 521(Pt 2):327–336. doi:10.1111/j.1469-7793.1999.00327.x
Yang J (1990) Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J Gen Physiol 96:1177–1198. doi:10.1085/jgp.96.6.1177
Corringer PJ, Bertrand S, Galzi JL et al (1999) Mutational analysis of the charge selectivity filter of the alpha7 nicotinic acetylcholine receptor. Neuron 22:831–843. doi:10.1016/S0896-6273(00)80741-2
Lee DJS, Keramidas A, Moorhouse AJ et al (2003) The contribution of proline 250 (P-2’) to pore diameter and ion selectivity in the human glycine receptor channel. Neurosci Lett 351:196–200. doi:10.1016/j.neulet.2003.08.005
Keramidas A, Moorhouse AJ, Pierce KD et al (2002) Cation-selective mutations in the M2 domain of the inhibitory glycine receptor channel reveal determinants of ion-charge selectivity. J Gen Physiol 119:393–410. doi:10.1085/jgp.20028552
Keramidas A, Moorhouse AJ, Schofield PR et al (2004) Ligand-gated ion channels: mechanisms underlying ion selectivity. Prog Biophys Mol Biol 86:161–204. doi:10.1016/j.pbiomolbio.2003.09.002
Akabas MH, Stauffer DA, Xu M et al (1992) Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science 258:307–310. doi:10.1126/science.1384130
Akabas MH, Kaufmann C, Archdeacon P et al (1994) Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron 13:919–927. doi:10.1016/0896-6273(94)90257-7
Wilson GG, Karlin A (1998) The location of the gate in the acetylcholine receptor channel. Neuron 20:1269–1281. doi:10.1016/S0896-6273(00)80506-1
Wilson G, Karlin A (2001) Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci USA 98:1241–1248. doi:10.1073/pnas.031567798
Xu M, Akabas MH (1993) Amino acids lining the channel of the γ-aminobutyric acid type A receptor identified by cysteine substitution. J Biol Chem 268:21505–21508
Xu M, Akabas MH (1996) Identification of channel-lining residues in the M2 membrane-spanning segment of the GABAA receptor α1 subunit. J Gen Physiol 107:195–205. doi:10.1085/jgp.107.2.195
Reeves DC, Goren EN, Akabas MH et al (2001) Structural and electrostatic properties of the 5-HT3 receptor pore revealed by substituted cysteine accessibility mutagenesis. J Biol Chem 276:42035–42042. doi:10.1074/jbc.M106066200
Panicker S, Cruz H, Arrabit C et al (2002) Evidence for a centrally located gate in the pore of a serotonin-gated ion channel. J Neurosci 22:1629–1639
Shan Q, Haddrill JL, Lynch JW (2002) Comparative surface accessibility of a pore-lining threonine residue (T6’) in the glycine and GABAA receptors. J Biol Chem 277:44845–44853. doi:10.1074/jbc.M208647200
Roberts DD, Lewis SD, Ballou DP et al (1986) Reactivity of small thiolate anions and cysteine-25 in papain toward methyl methanethiosulfonate. Biochem 25:5595–5601. doi:10.1021/bi00367a038
Shan Q, Haddrill JL, Lynch JW (2001) A single β subunit M2 domain residue controls the picrotoxin sensitivity of αβ heteromeric glycine receptor chloride channels. J Neurochem 76:1109–1120. doi:10.1046/j.1471-4159.2001.00124.x
Mascia MP, Gong DH, Eger EI 2nd et al (2000) The anesthetic potency of propanol and butanol versus propanethiol and butanethiol in alpha1 wild type and alpha1(S267Q) glycine receptors. Anesth Analg 91:1289–1293. doi:10.1097/00000539-200011000-00044
Lobo IA, Mascia MP, Trudell JR et al (2004) Channel gating of the glycine receptor changes accessibility to residues implicated in receptor potentiation by alcohols and anesthetics. J Biol Chem 279:33919–33927. doi:10.1074/jbc.M313941200
Cymes GD, Grosman C, Auerbach A (2002) Structure of the transition state of gating in the acetylcholine receptor channel pore: a phi-value analysis. Biochem 41:5548–5555. doi:10.1021/bi011864f
Goren EN, Reeves DC, Akabas MH (2004) Loose protein packing around the extracellular half of the GABAA receptor β1 subunit M2 channel-lining segment. J Biol Chem 279:11198–11205. doi:10.1074/jbc.M314050200
Horenstein J, Wagner DA, Czajkowski C et al (2001) Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat Neurosci 4:477–485
Akabas MH (2004) GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol 62:1–43. doi:10.1016/S0074-7742(04)62001-0
Filatov GN, Aylwin ML, White MM (1993) Selective enhancement of the interaction of curare with the nicotinic acetylcholine receptor. Mol Pharmacol 44:237–241
Labarca C, Nowak MW, Zhang H et al (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376:514–516. doi:10.1038/376514a0
Bertrand D, Galzi JL, Devillers-Thiery A et al (1993) Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci USA 90:6971–6975. doi:10.1073/pnas.90.15.6971
Palma E, Mileo AM, Eusebi F et al (1996) Threonine-for-leucine mutation within domain M2 of the neuronal alpha(7) nicotinic receptor converts 5-hydroxytryptamine from antagonist to agonist. Proc Natl Acad Sci USA 93:11231–11235. doi:10.1073/pnas.93.20.11231
Palma E, Fucile S, Barabino B et al (1999) Strychnine activates neuronal alpha7 nicotinic receptors after mutations in the leucine ring and transmitter binding site domains. Proc Natl Acad Sci USA 96:13421–13426. doi:10.1073/pnas.96.23.13421
Pascual JM, Karlin A (1998) State-dependent accessibility and electrostatic potential in the channel of the acetylcholine receptor. J Gen Physiol 111:717–739. doi:10.1085/jgp.111.6.717
Mascia MP, Mihic SJ, Valenzuela CF et al (1996) A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol 50:402–406
Yang Z, Webb TI, Lynch JW (2007) Closed-state cross-linking of adjacent β1 subunits in α1β1 GABAA receptors via introduced 6’ cysteines. J Biol Chem 282:16803–16810. doi:10.1074/jbc.M611555200
Imoto K, Busch C, Sakmann B et al (1988) Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335:645–648. doi:10.1038/335645a0
Imoto K, Konno T, Nakai J et al (1991) A ring of uncharged polar amino acids as a component of channel constriction in the nicotinic acetylcholine receptor. FEBS Lett 289:193–200. doi:10.1016/0014-5793(91)81068-J
Acknowledgments
We thank Kerrie Pierce, Anna Scimone, Cheryl Handford and Irene Michas for excellent technical assistance. This work was supported by the National Health and Medical Research Council of Australia (Project grants 230806 and 455310 and Research Fellowship 157209). N.L.A was the recipient of an Australian Postgraduate Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Absalom, N.L., Schofield, P.R. & Lewis, T.M. Pore Structure of the Cys-loop Ligand-gated Ion Channels. Neurochem Res 34, 1805–1815 (2009). https://doi.org/10.1007/s11064-009-9971-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9971-2