Abstract
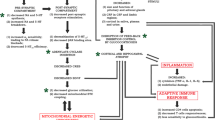
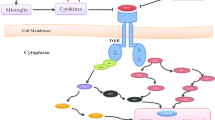
Major depressive disorder (MDD) is a common and devastating psychiatric disorder characterized by persistent low mood, cognitive disorder, and impaired social function. Despite its complex mechanisms, increasing evidence has identified the involvement of neurotrophic factors, inflammatory cytokines, the hypothalamus-pituitary-adrenal axis, and glutamate receptors in the pathophysiology of this illness. The present review synthesizes recent research achievements to define the network between different hypotheses of MDD and to understand which part is most pivotal for its pathogenesis. By integrating MDD-related signal pathways, we highlight brain-derived neurotrophic factor (BDNF) dysfunction and increased apoptosis as the final common cascades, and new therapeutic strategies aiming to enhance BDNF function have been shown to exert a rapid and effective antidepressant action.
Similar content being viewed by others
References
Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006, 49: 837–845.
Mathers C, Fat DM, Boerma JT. The global burden of disease: 2004 update. World Health Organization, 2008.
Brundtland GH. From the World Health Organization. Mental health: new understanding, new hope. JAMA 2001, 286: 2391.
Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx 2005, 2: 590–611.
Block SG, Nemeroff CB. Emerging antidepressants to treat major depressive disorder. Asian J Psychiatr 2014, 12C: 7–16.
Montes JM, Ferrando L, Saiz-Ruiz J. Remission in major depression with two antidepressant mechanisms: results from a naturalistic study. J Affect Disord 2004, 79: 229–234.
Bortolozzi A, Castane A, Semakova J, Santana N, Alvarado G, Cortes R, et al. New antidepressant strategy based on acute siRNA silencing of 5-HT1A autoreceptors. Mol Psychiatry 2012, 17: 567.
Assie MB, Lomenech H, Ravailhe V, Faucillon V, Newman-Tancredi A. Rapid desensitization of somatodendritic 5-HT1A receptors by chronic administration of the high-efficacy 5-HT1A agonist, F13714: a microdialysis study in the rat. Br J Pharmacol 2006, 149: 170–178.
Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2475–2484.
Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol 2011, 9: 530–552.
Holtzman DM, Mobley WC. Neurotrophic factors and neurologic disease. West J Med 1994, 161: 246–254.
Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res 2001, 120: 87–95.
Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002, 109: 143–148.
Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry 2000, 48: 732–739.
Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995, 15: 7539–7547.
Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 1997, 56: 131–137.
Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella MC, et al. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology 2008, 57: 139–145.
Dwivedi Y. Brain-derived neurotrophic factor in suicide pathophysiology. In: Dwivedi Y (ed.). The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press, 2012, Chapter 8.
Kanellopoulos D, Gunning FM, Morimoto SS, Hoptman MJ, Murphy CF, Kelly RE, et al. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriatr Psychiatry 2011, 19: 13–22.
Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 2008, 153Suppl 1: S310–324.
Altar CA. Neurotrophins and depression. Trends Pharmacol Sci 1999, 20: 59–61.
Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006, 59: 1116–1127.
Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 2005, 12: 1329–1343.
Lu B. BDNF an d activity-dependent synaptic modulation. Learn Mem 2003, 10: 86–98.
Duncan WC, Jr., Zarate CA, Jr. Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep 2013, 15: 394.
Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 2008, 28: 4088–4095.
Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep 2007, 30: 129–139.
Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 2012, 72: e27–28.
Bachmann V, Klein C, Bodenmann S, Schafer N, Berger W, Brugger P, et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 2012, 35: 335–344.
Barker PA. Whither proBDNF? Nat Neurosci 2009, 12: 105–106.
Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol 2013, 100:15–29.
Kikuchi-Utsumi K, Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neurosci Lett 2008, 440: 19–22.
Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse 2012, 66: 630–639.
Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci 1996, 16: 1193–1202.
Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res 2009, 198: 472–476.
Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav 1998, 61: 247–252.
Kobayashi T, Matsuno K, Murai M, Mita S. Sigma 1 receptor subtype is involved in the facilitation of cortical dopaminergic transmission in the rat brain. Neurochem Res 1997, 22: 1105–1109.
Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 1998, 82: 443–468.
Kulkarni SK, Dhir A. sigma-1 receptors in major depression and anxiety. Expert Rev Neurother 2009, 9: 1021–1034.
Han QQ, Yu J. Inflammation: a mechanism of depression? Neurosci Bull 2014, 30: 515–523.
Hashmi AM, Butt Z, Umair M. Is depression an inflammatory condition? A review of available evidence. J Pak Med Assoc 2013, 63:899–906.
Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal 2012, 5: pe45.
Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry 2014, 48: 102–111.
Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp 2008, 289: 222–233; discussion 233–240.
Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 2005, 135: 659–678.
Na KS, Lee KJ, Lee JS, Cho YS, Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2014, 48: 79–85.
Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007, 21: 9–19.
Li WZ, Li WP, Yao YY, Zhang W, Yin YY, Wu GC, et al. Glucocorticoids increase impairments in learning and memory due to elevated amyloid precursor protein expression and neuronal apoptosis in 12-month old mice. Eur J Pharmacol 2010, 628:108–115.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009, 65: 732–741.
Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 2008, 432: 232–236.
Maciejewski PK, Rothman DL. Proposed cycles for functional glutamate trafficking in synaptic neurotransmission. Neurochem Int 2008, 52: 809–825.
Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant’Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr 2008, 30: 243–245.
Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, et al. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem 2009, 108: 1251–1265.
Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 2002, 5: 405–414.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73: 962–977.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007, 32: 1888–1902.
Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011, 224: 107–111.
Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res 2013, 238: 48–52.
Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother 2011, 11: 33–36.
Monteggia LM, Gideons E, Kavalali ET. The role o f eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 2013, 73: 1199–1203.
Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 2011, 16: 1068–1070.
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res 2013, 91: 30–41.
Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 2002, 7: 837–844.
Zugno AI, Juliao RF, Budni J, Volpato AM, Fraga DB, Pacheco FD, et al. Rivastigmine reverses cognitive deficit and acetylcholinesterase activity induced by ketamine in an animal model of schizophrenia. Metab Brain Dis 2013, 28: 501–508.
Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology 2013, 65: 29–38.
Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol 2012, 15: 429–434.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacol 2013, 38: 729–742.
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 2014, 23: 243–254.
Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun 2002, 16: 622–653.
Kumamaru E, Numakawa T, Adachi N, Kunugi H. Glucocorticoid suppresses BDNF-stimulated MAPK/ERK pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett 2011, 585: 3224–3228.
Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology 2014, 42: 165–177.
Zhang J, Fan Y, Li Y, Zhu H, Wang L, Zhu MY. Chronic social defeat up-regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid-dependent manner. J Neurochem 2012, 123: 1054–1068.
Kling MA, Coleman VH, Schulkin J. Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety 2009, 26: 641–649.
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377: 68–71.
Jing L, Bu M. Role of macrophage migration inhibitory factor in glucocorticoid release and glucocorticoid receptor function in rats. Ann Clin Lab Sci 2011, 41: 14–19.
Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain Behav Immun 2010, 24: 1202–1208.
Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison K J, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacol 2013, 38: 377–385.
Conboy L, Varea E, Castro JE, Sakouhi-Ouertatani H, Calandra T, Lashuel HA, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry 2011, 16: 533–547.
Moon HY, Kim SH, Yang YR, Song P, Yu HS, Park HG, et al. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc Natl Acad Sci U S A 2012, 109: 13094–13099.
Quera Salva MA, Hartley S, Barbot F, Alvarez JC, Lofaso F, Guilleminault C. Circadian rhythms, melatonin and depression. Curr Pharm Des 2011, 17: 1459–1470.
Murray G. Diurnal mood variation in depression: a signal of disturb ed circadian function? J Affect Disord 2007, 102: 47–53.
Suchecki D, Tiba PA, Machado RB. REM Sleep Rebound as an Adaptive Response to Stressful Situations. Front Neurol 2012, 3: 41.
Beck-Friis J, Kjellman BF, Aperia B, Unden F, von Rosen D, Ljunggren JG, et al. Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand 1985, 71: 319–330.
Khaleghipour S, Masjedi M, Ahade H, Enayate M, Pasha G, Nadery F, et al. Morning and nocturnal serum melatonin rhythm levels in patients with major depressive disorder: an analytical cross-sectional study. Sao Paulo Med J 2012, 130: 167–172.
Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011, 35: 1569–1574.
Gulec M, Selvi Y, Boysan M, Aydin A, Besiroglu L, Agargun MY. Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord 2011, 134: 257–265.
Campino C, Valenzuela FJ, Torres-Farfan C, Reynolds HE, Abarzua-Catalan L, Arteaga E, et al. Melatonin exerts direct inhibitory actions on ACTH responses in the human adrenal gland. Horm Metab Res 2011, 43: 337–342.
Konakchieva R, Mitev Y, Almeida OF, Patchev VK. Chronic melatonin treatment counteracts glucocorticoid-induced dysregulation of the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology 1998, 67: 171–180.
Avery D, Lenz M, Landis C. Guidelines for prescribing melatonin. Ann Med 1998, 30: 122–130.
Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol 2008, 23: 571–585.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, S., Huang, S. & Hao, W. New hypothesis and treatment targets of depression: an integrated view of key findings. Neurosci. Bull. 31, 61–74 (2015). https://doi.org/10.1007/s12264-014-1486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-014-1486-4