Abstract
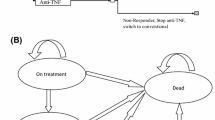
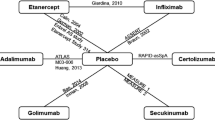
As part of the National Institute for Health and Clinical Excellence (NICE) single technology appraisal (STA) process, the Evidence Review Group (ERG) produced a report to comment on the clinical and cost effectiveness of golimumab (Simponi®, Merck Sharp & Dohme) for the treatment of ankylosing spondylitis (AS) relative to other comparators as presented in the manufacturer’s submission (MS) to NICE. The population was those with active disease who had not responded to conventional therapy. The specified comparators were conventional care and two other tumour necrosis factor alpha (TNF-α) inhibitors (adalimumab and etanercept). Outcomes to be considered were disease activity, functional capacity, disease progression, adverse effects of treatment and health-related quality of life (HR-QOL). There were no head-to-head trials comparing TNF-α inhibitors. The submission included one trial of golimumab versus placebo (the GO-RAISE trial) and additionally seven placebo-controlled randomized controlled trials (RCTs) of other TNF-α inhibitor agents (five with etanercept, and two with adalimumab). The results of these trials were generally a statistically significant improvement from each of the TNF-α inhibitors. A Bayesian mixed treatment comparison (MTC) showed there was generally overlap in the 95 % credible intervals (CrIs) between the TNF-α inhibitors. Exceptions included a greater risk of discontinuation of treatment for golimumab than for etanercept (relative risk [RR] 4.30; 95 % CrI 1.01–18.50). The cost-effectiveness analysis (CEA) compared all of these TNF-α inhibitors. Relative effectiveness was informed only by RR of response (proportion achieving at least a 50 % improvement in Bath AS Disease Activity Index [BASDAI] score; BASDAI50) from the MTC. In the base-case analysis, the incremental cost-effectiveness ratio (ICER) of golimumab versus conventional care was £26,597 and adalimumab and etanercept were extendedly dominated by golimumab. The manufacturer concluded that golimumab is a cost-effective treatment option. Generally, the ERG agreed with the MTC analyses. The main problem was that the MS used data from one trial, which included a period of cross-over. The ERG found some problems with the CEA model, mainly that it did not allow for comparison of TNF-α inhibitor sequences and did not use MTC estimates for treatment discontinuation or adverse events (AEs). The ERG could not correct the sequencing problem, but re-ran the CEA with discontinuations and AEs estimated from the MTC and using the correct trial data. The results of the ERG analysis were that golimumab was extendedly dominated by etanercept, and the preferred treatment was either conventional treatment or etanercept, depending on the ICER threshold. Uncertainty was also substantial. NICE issued guidance (technology appraisal [TA] 233), which recommended golimumab according to the indications described in TA143 for etanercept and adalimumab, i.e. as first-line therapy among the TNF-α inhibitors unless patients are intolerant to one or both alternatives. Given the factors cited by NICE for their decision, the ERG recommends that there should be greater clarity in the NICE methods guidance on handling uncertainty in CEAs as well as the incorporation of benefit from process of care.

Similar content being viewed by others
References
Yang H, Craig D, Epstein D, Bojke L, Light K, Bruce IN, et al. Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(4):257–70.
Sculpher M. Single technology appraisal at the UK National Institute for Health and Clinical Excellence: a source of evidence and analysis for decision making internationally. Pharmacoeconomics. 2010;28(5):347–9.
Rodgers M, Griffin S, Paulden M, et al. Alitretinoin for severe chronic hand eczema: a NICE single technology appraisal. Pharmacoeconomics. 2010;28(5):351–62.
Bagust A, Greenhalgh J, Boland A, et al. Cetuximab for recurrent and/or metastatic squamous cell carcinoma of the head and neck: a NICE single technology appraisal. Pharmacoeconomics. 2010;28(6):439–48.
Stevenson M, Pandor A. Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(2):133–40.
Scotland G, Waugh N, Royle P, et al. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(11):951–61.
Dickson R, Bagust A, Boland A, et al. Erlotinib monotherapy for the maintenance treatment of non-small cell lung cancer after previous platinum-containing chemotherapy: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(12):1051–62.
McKenna C, Maund E, Sarowar M, et al. Dronedarone for the treatment of atrial fibrillation: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(1):35–46.
Holmes M, Carroll C, Papaioannou D. Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(2):137–46.
Boyers D, Jia X, Jenkinson D, et al. Eltrombopag for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(6):483–95.
Burch J, Griffin S, McKenna C, Walker S, Paton J, Wright K, et al. Omalizumab for severe persistent asthma in children aged 6–11 years: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(11):991–1004.
Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(12):1119–32.
Kilonzo M, Hislop J, Elders A, Fraser C, Bissett D, McClinton S, et al. Pazopanib for the first-line treatment of patients with advanced and/or metastatic renal cell carcinoma: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(1):15–24.
Craig D, Rice S, Paton F, et al. Retigabine for the adjunctive treatment of adults with partial onset seizures in epilepsy with and without secondary generalisation: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(2):101–10.
Spackman E, Rice S, Norman G, Suh D-C, Eastwood A, Palmer S. Trastuzumab for the treatment of HER2 positive metastatic gastric cancer: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(3):185–94.
Rafia R, Simpson E, Stevenson M, Papaioannou D. Trabectedin for the treatment of advanced metastatic soft tissue sarcoma: a NICE single technology appraisal. Pharmacoeconomics. 2013. doi:10.1007/s40273-013-0044-7.
Simpson EL, Fitzgerald P, Evans P, et al. Bivalirudin for the treatment of ST-segment elevation myocardial infarction: a NICE single technology appraisal. Pharmacoeconomics. 2013. doi:10.1007/s40273-013-0036-7.
Greenhalgh J, Bagust A, Boland A, Blundell M, Oyee J, Beale S, Dundar Y, Hockenhull J, Proudlove C, Chu P. Rituximab for the first-line maintenance treatment of follicular non-Hodgkin’s lymphoma: a NICE single technology appraisal. Pharmacoeconomics. 2013. doi:10.1007/s40273-013-0043-8.
Tosh J, Archer R, Davis S, et al. Golimumab for the treatment of rheumatoid arthritis after the failure of previous disease-modifying anti-rheumatic drugs: a NICE single technology appraisal. Pharmacoeconomics. In press.
Kearns B, Lloyd-Jones M, Stevenson M, Littlewood C. Cabazitaxel for the second-line treatment of metastatic hormone refractory prostate cancer: a NICE single technology appraisal. Pharmacoeconomics. 2013. doi:10.1007/s40273-013-0050-9.
Faria R, Spackman E, Burch J, Corbacho B, Todd D, Pepper C, et al. Dabigatran for the prevention of stroke and systemic embolism in atrial fibrillation: a NICE single technology appraisal. PharmacoEconomics. In press.
Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61–6.
Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63(5):535–43.
Boyer GS, Templin DW, Bowler A, Lawrence RC, Everett DF, Heyse SP, et al. A comparison of patients with spondyloarthropathy seen in specialty clinics with those identified in a communitywide epidemiologic study. Has the classic case misled us? Arch Intern Med. 1997;157(18):2111–7.
Sieper J, Rudwaleit M. How early should ankylosing spondylitis be treated with tumour necrosis factor blockers? Ann Rheum Dis. 2005;64(Suppl 4):iv61–4.
Chary-Valckenaere I, d’Agostino MA, Loeuille D. Role for imaging studies in ankylosing spondylitis. Jt Bone Spine. 2011;78(2):138–43.
Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61(Suppl 3):iii8–18.
Carette S, Graham D, Little H, Rubenstein J, Rosen P. The natural disease course of ankylosing spondylitis. Arthritis Rheum. 1983;26(2):186–90.
Gran JT, Skomsvoll JF. The outcome of ankylosing spondylitis: a study of 100 patients. Br J Rheumatol. 1997;36(7):766–71.
Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60(3):717–27.
Ward MM. Predictors of the progression of functional disability in patients with ankylosing spondylitis. J Rheumatol. 2002;29(7):1420–5.
Dagfinrud H, Hagen Kåre B, Kvien Tore K. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No.: CD002822. doi:10.1002/14651858.CD002822.pub3.
Chen J, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD004524. doi:10.1002/14651858.CD004524.pub3.
Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No.: CD004800. doi:10.1002/14651858.CD004800.pub2.
McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 2007;11(28):1–158, iii–iv.
Centocor Inc. Clinical study report: a multicenter, randomized, double-blind, placebo-controlled trial of golimumab, a fully human TNF-α inhibitor α monoclonal antibody, administered subcutaneously, in subjects with active ankylosing spondylitis (GO-RAISE), 2007. Horsham: Centocor; 2007.
Inman R, Davis JJ, Heijde D, Diekman L, Sieper J, Kim S, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58(11):3402–12.
van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54(7):2136–46.
Van der Heijde D, Schiff M, Sieper J, Kivitz A, Wong R, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Annals Rheum Dis. 2009;68:922–9.
Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48(6):1667–75.
Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–56.
Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2006;65:442–52.
Calin A, Dijkmans B, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004;63(12):1594–600.
Davis JJ, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg D, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48(11):3230–6.
van der Heijde D, Da Silva J, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65(12):1572–7.
Maksymowych W, Rahman P, Keystone E, Wong R. Efficacy of adalimumab in active ankylosing spondylitis (AS): results of the Canadian AS study. Arthritis Rheum. 2005;52(Suppl):S217.
European Medicines Agency. Annex I: summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000992/WC500052368.pdf. Accessed 21 Mar 2013.
Connock M, Tubeuf S, McCabe C, Jobanputra P, Hassan A, Bayliss S, et al. Golimumab for the treatment of adults with active Ankylosing spondylitis whose response to conventional therapy has been inadequate: a Single Technology Appraisal. Birmingham: WMHTAC, University of Birmingham; 2010.
National Institute for Health and Clinical Excellence. Ankylosing spondylitis: golimumab. London: NICE. http://guidance.nice.org.uk/TA/Wave19/48. Accessed 27 Jun 2012.
Schering-Plough Ltd. Golimumab for the treatment of Ankylosing Spondylitis (AS)—Response to request for clarification from the ERG. Hoddesdon: Schering-Plough Ltd (part of MSD); 2011.
National Institute for Health and Clinical Excellence. Single Technology Appraisal: specification for manufacturer/sponsor submission of evidence. London: NICE; 2009.
National Institute for Health and Clinical Excellence. Ankylosing spondylitis—adalimumab, etanercept and infliximab: Final appraisal determination. London: NICE; 2007.
Department of Health. NHS reference costs 2007-08. London: Department of Health; 2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945. Accessed 21 Mar 2013.
Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–37.
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: NICE; 2008.
National Institute for Health and Clinical Excellence. Golimumab for the treatment of ankylosing spondylitis (TA233). London: NICE; 2011.
National Institute for Health and Clinical Excellence. NICE’s response to the Kennedy study. London: NICE; 2010. http://www.nice.org.uk/aboutnice/howwework/researchanddevelopment/KennedyStudyNICEResponse.jsp. Accessed 21 March 2013.
Riemsma R, Joore M, Van Asselt T, Armstrong N, Misso K, Manning N, et al. Golimumab for the treatment of ankylosing spondylitis: a single technology appraisal. York: Kleijnen Systematic Reviews Ltd.; 2011.
Acknowledgments
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (project number 08/205/01). See the HTA Programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The views and opinions expressed are the authors’ and do not necessarily reflect those of NICE or the Department of Health. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author Contributions
Rob Riemsma acted as project lead and systematic reviewer on this appraisal, critiqued the clinical effectiveness methods and evidence, and contributed to the writing of the article. Nigel Armstrong acted as health economist on this assessment, critiqued the manufacturer’s economic evaluation and contributed to the writing of the article. Kate Misso critiqued the search methods in the submission and contributed to the writing of the article. Nathan Manning acted as systematic reviewer, critiqued the clinical effectiveness methods and evidence, and contributed to the writing of the article. Thea van Asselt acted as health economist on this appraisal, critiqued the manufacturer’s economic evaluation and contributed to the writing of the article. Manuela Joore acted as health economic project lead, critiqued the manufacturer’s economic evaluation and contributed to the writing of the article. Florian Tomini acted as health economist on this appraisal, critiqued the manufacturer’s economic evaluation and contributed to the writing of the article. Jos Kleijnen critiqued the manufacturer’s definition of the decision problem and their description of the underlying health problem and current service provision, contributed to the writing of the article and supervised the project. Nigel Armstrong acts as guarantor for the overall content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, N., Joore, M., van Asselt, T. et al. Golimumab for the Treatment of Ankylosing Spondylitis: A NICE Single Technology Appraisal. PharmacoEconomics 31, 415–425 (2013). https://doi.org/10.1007/s40273-013-0049-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0049-2