Abstract
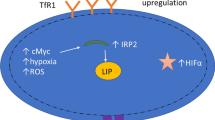
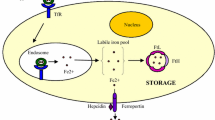
Iron homeostasis is crucial to normal cell metabolism, and its deficiency or excess is associated with numerous disease states. The association of increased iron load with cancer may be due to several factors including free radical production, reduction of the body's protective mechanism to combat oxidative stress, inhibition of immune systems, inhibition of essential nutrient functions, facilitation of cancer growth, suppression of antitumor actions of macrophages, and lowering of the ratio of T4–T8 positive lymphocytes. Antiproliferative effects of desferoxamine (DFO) both in vitro and in vivo are mediated by an intracellular pool of iron that is necessary for DNA synthesis rather than prevention of iron uptake from transferrin. Several clinical studies have shown it to have antitumor activity in the treatment of neuroblastoma, leukemia, bladder carcinoma, and hepatocellular carcinoma. Human neural tumor cells are susceptible to the effects of DFO. Continued study of DFO is necessary to further elucidate its antineoplastic profile and its use as an adjunct to current chemotherapy regimens. Given the lack of satisfactory treatment of central nervous system neoplasms, DFO could serve as an important tool in the management of such cancers.
Similar content being viewed by others
References
Beard JL, Connor JD, Jones B: Brain iron: location and function. Prog Food Nutr Sci 17(3): 183–221, 1993
Gabutti V, Piga A: Results of long-term iron-chelating therapy. Acta Haematol 95(1): 26–36, 1996
Richardson DR, Baker E: The uptake of iron and transferrin by the human melanoma cell. Biochim Biophys Acta 1053: 1–12, 1990
Richardson DR, Ponka P: The iron metabolism of the human neuroblastoma cell: lack of relationship between the efficacy of iron chelation and the inhibition of DNA synthesis. J Lab Clin Med 124(5): 660–671, 1994
Trinder D, Morgan EH, Baker E: The mechanisms of iron uptake by rat fetal hepatocytes. Hepatology 6: 852–858, 1986
Kuhn LC, Schulman HM, Ponka P. Iron-transferrin requirements and transferrin receptor expression in proliferating cells. In: Ponka P, Schulman HM, Woodwoth RC (eds) Iron Transport and Storage. CRC Press, Boca Raton, FL. 1990, pp. 141-191
Murray MT, White K, Munro HN: Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci 84: 7438–7442, 1987
Richardson DR, Ponka P: The molecular mechanism of the metabolism and transport of iron in normal and neoplastic cells. Biochem Biophys Acta 1331: 1–40, 1997
Richardson DR: Potential of iron chelators as effective antiproliferative agents. Can J Physiol Pharmacol 75(10–11): 1164–1180, 1997
Thelander L, Reichard P: The reduction of ribonucleotides. Annu Rev Biochem 48: 133–158, 1979
Youdim MB, Ben-Shachar D, Riederer P: Iron in brain function and dysfunction with emphasis on Parkinson's disease. Eur Neurol (Suppl 31)1: 34–40, 1991
Wright JA, Chan AK, Choy BK, Hurta RA, McClarty GA, Tagger AY: Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem Cell Biol 68: 1364–1371, 1990
Rao K, Harford JB, Rouault T, McClelland A, Ruddle FH, Klaus: Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol 6: 236–240, 1986
White K, Munro HN: Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem 263: 8938–8942, 1988
Alcantara O, Javors M, Boldt DH: Induction of protein kinase C-mRNA in cultured lymphoblastoid T-cells by iron transferrin but not by soluble iron. Blood 77: 1290–1297, 1991
Alcantara O, Obeid L, Hannun Y, Ponka P, Boldt DH: Regulation of protein kinase C (PKC) expression by iron: effect of different iron compounds on PKC-beta and PKC-alpha gene expression and role of the 5′ flanking region of the PKC-beta gene in the response to ferric transferrin. Blood 84: 3610–3617, 1994
Alcantara O, Reddy SV, Roodman GD, Boldt DH: Transcriptional regulation of the tartate-resistant acid phosphatase (TRAP) gene by iron. Biochem J 298: 421–425, 1994
Hoppe J, Schumacher L, Eichner W, Weich HA: The long 3′-untranslated regions of the PDGF-A and B mRNAs are only distantly related. FEBS Lett 223: 243–246, 1987
Weiss G, Werner-Falmayer G, Werner ER, Grunewald K, Wachter: Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 180: 969–976, 1994
Boldt DH: New perspectives on iron: an introduction. Am J Med Sci 318(4): 207–212, 1999
Dorfman DM, Wilson DB, Bruns GA, Orkin SH: Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J Biol Chem 267: 1279–1285, 1992
Dolce W, Iacobazzi V, Palmieri F, Walker JE: The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms. J Biol Chem 269: 10451–10460, 1994
Fleckenstein E, Dirks W, Dehmel U, Drexler HG: Cloning and characterization of the human tartate-resistant acid phosphatase (TRAP) gene. Leukemia 10: 637–643, 1996
Krauss S, Franke WW: Organization and sequence of the gene encoding cytokeratin 8. Gene 86: 241–249, 1990
Keberle H: The biochemistry of desferrioxamine and its relation to iron metabolism. Ann NY Acad Sci 119: 758–768, 1964
Fielding J: Differential ferrioxamine test for measuring chelatable body iron. J Clin Pathol 18: 88–97, 1965
Donfrancesco A, Deb G, De Sio L, Cozza R, Castellano A: Role of deferoxamine in tumor therapy. Acta Haematol 95(1): 66–69, 1996
Kontogiorghes GJ: Comparative efficacy and toxicity of desferrioxamine, deferipone and other iron and aluminum chelating drugs. Toxicol Lett 80 1–18, 1995
Ijichi A, Sakuma S, Tofilon PJ: Hypoxia-induced vascular endothelial growth factor expression in normal rat astrocyte cultures. Glia 14(2): 87–93, 1995
Richardson DR, Baker E: Two saturable mechanisms of iron uptake from transferrin in human melanoma cells: the effect of transferrin concentrations, chelators and metabolic probes on transferrin and iron uptake. J Cell Physiol 161: 160–168, 1995
Nyholm S, Mann GJ, Johansson AG, Bergeron RJ, Graslund A, Thelander L: Role of ribonucleotide reductase in inhibition of mammalian cell cycle growth by potent iron chelators. J Biol Chem 268: 26200–26205, 1993
Renton FJ, Jeitner TM: Cell cycle-dependent inhibition of the proliferation of human neural tumor cell lines by iron chelators. Biochem Pharmacol 51(11): 1553–1561, 1996
Zheng Y, Connor JR: Screening of transcriptionally regulated genes following iron chelation in human astrocytoma cells. Biochem Biophys Res Commun 264: 709–713, 1999
Brodie C, Siriwardana G, Lucas J, Schleicher R, Terada N, Szepesi A, Gelfand E, Seligman P: Neuroblastoma sensitivity to growth inhibition by deferrioxamine: evidence for a block in G1 phase of the cell cycle. Cancer Res 53(17): 3968–3975, 1993
Toyokuni S: Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med 20(4): 553–566, 1996
Weinberg ED: Roles of iron in neoplasia. Promotion, prevention, and therapy. Biol Trace Elem Res 34(2): 123–140, 1992
McCord JM: Effects of positive iron status at a cellular level. Nutr Rev 54(3): 85–88, 1996
Selig RA, White L, Gramacho C, Sterling-Levis K, Fraser IW, Naidoo D: Failure of iron chelators to reduce tumor growth in human neuroblastoma xenografts. Cancer Res 58(3): 473–478, 1998
Brodeur GM, Seeger RC, Scwab M, Varmus HE, Bishop JM: Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224(4653): 1121–1124, 1984
Frantz CN, Eskenazi AE, and Ocerman D: Iron chelation cancer therapy for neuroblastoma. Proc Int Conf Bioiron Asheville, N.C. 1995, p. 195
Frantz CN, Iyer J, Frick KK, Eskenazi AE, Derg PE: Iron and N-myc expression in neuroblastoma. Neuroblastoma Research Meeting, Philadelphia, 1993
Estrov Z, Tawa A, Wang XH, Dube ID, Sulh H, Cohen A, Gelfand EW, Freedman MH: In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood 69(3): 757–761, 1987
Hann HW, Stahlhut MW, Rubin R, Maddrey WC: Antitumor effect of deferoxamine on human hepatocellular carcinoma growing in athymic nude mice. Cancer 70(8): 2051–2056, 1992
Seligman PA, Schleicher RB, Siriwardana G, Domenico J, Gelfand EW: Effects of agents that inhibit cellular iron incorporation on bladder cancer cell proliferation. Blood 182(5): 1608–1617, 1993
Frantz CN, Bernstein M, Castelberry R, Vietti T: Dose escalation of desferrioxamine (DFO) in children with refractory neuroblastoma: A Pediatric Oncology Group Study. Proc Am Soc Clin Oncol 416, 1994
Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S: Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood 88(2): 705–713, 1996
Blatt J: Deferoxamine in children with recurrent neuroblastoma. Anticancer Res 14(5B): 2109–2112, 1994
Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L: Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res 50(16): 4929–4930, 1990
Donfrancesco A, Deb G, Dominici C, Angioni A, Caniglia M, De Sio L, Fidani P, Amici A, Helson L: Deferoxamine, cyclophosphamide, etoposide, carboplatin, and thiotepa (D-CECaT): a new cytoreductive chelation-chemotherapy regimen in patients with advanced neuroblastoma. Am J Clin Oncol 15(4): 319–322, 1992
Donfrancesco A, De Bernardi B, Carli M, Mancini A, Nigro M, De Sio L, Casale F, Bagnulo S, Helson L, Deb G: Deferoxamine followed by cyclophosphamide, etoposide, carboplatin, thiotepa, induction regimen in advanced neuroblastoma: preliminary results. Italian Neuroblastoma Cooperative Group. Eur J Cancer 31A(4): 612–615, 1995
Donfrancesco A, Deb G, Dominici C, De Sio L, Inserra A, Boglino C, Takahashi M, Uchino J, Helson L: D-CECaT as preoperative chemotherapy for unresectable neuroblastoma in children over one year of age. Anticancer Res 15(5B): 2347–2350, 1995
Kaplinsky C, Schvartzmayer S, Goshen Y: Desferal Treatment in 3 Leukemic Patients, SIOP Meeting, Trondheim, 1988 (Abstract)
Valle P, Timeus F, Piglione M, Rosso P, di Montezemolo LC, Crescenzio N, Marranca D, Ramenghi U: Effect of different exposures to desferrioxamine on neuroblastoma cell lines. Pediatr Hematol Oncol 12(5): 439–446, 1995
Blatt J, Stitely S: Antineuroblastoma activity of desferoxamine in human cell lines. Cancer Res 47(7): 1749–1750, 1987
Becton DL, Bryles P: Deferoxamine inhibition of human neuroblastoma viability and proliferation. Cancer Res 48(24Pt 1): 7189–7192, 1988
Becton DL, and Roberts B: Antileukemic effects of desferoxamine on human myeloid leukemia cell lines. Cancer Res 49: 4809–4812, 1989
Summers MR, Jacobs A, Tudway D, Perera P, Ricketts C: Studies of desferrioxamine and ferrioxamine metabolism in normal and iron-loaded subjects. Br J Hematol 42: 547–555, 1979
Richardson DR, Tran E, Ponka P: The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood 86: 4295–4306, 1995
Richardson DR, Milnes K: The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. II. The mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood 3025-3038, 1997
Lee YS, Wurster RD: Deferoxamine-induced cytotoxicity in human neuronal cell lines: protection by free radical scavengers. Toxicol Lett 78(1): 67–71, 1995
Voest EE, Vreugdenhil G, Marx JJ: Iron-chelating agents in non-iron overload conditions. Ann Int Med 120(6): 490–499, 1994
Beerepoot LV, Shima DT, Kuroki M, Yeo KT, Voest EE: Up-regulation of vascular endothelial growth factor production by iron chelators. Cancer Res 56(16): 3747–3751, 1996
Sinor AD, Irvin SM, Cobbs CS, Chen J, Graham SH, Greenberg DA: Hypoxic induction of vascular endothelial growth factor (VEGF) protein in astroglial cultures. Brain Res 812(1–2): 289–291, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dayani, P.N., Bishop, M.C., Black, K. et al. Desferoxamine (DFO) – Mediated Iron Chelation: Rationale for a Novel Approach to Therapy for Brain Cancer. J Neurooncol 67, 367–377 (2004). https://doi.org/10.1023/B:NEON.0000024238.21349.37
Issue Date:
DOI: https://doi.org/10.1023/B:NEON.0000024238.21349.37