Abstract
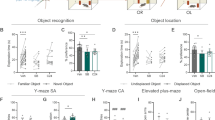
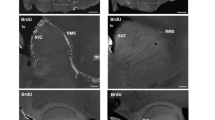
The peptide nociceptin (also named orphanin FQ) acts in the brain to produce various pharmacological effects, including hyperalgesia and hypolocomotion1,2. The nociceptin receptor uses guanine-nucleotide-binding proteins to mediate the inhibition of adenylyl cyclase, the activation of potassium channels and inhibition of calcium channels3. It has been shown using knockout mice that the nociceptin receptor is not required for regulation of nociceptive responses or locomotion activity, but modulates the auditory function4. Here we show that mice lacking the nociceptin receptor possess greater learning ability and have better memory than control mice. Histological analysis revealed the expression of both the nociceptin precursor and the nociceptin receptor in the hippocampus, thought to take part in aspects of learning and memory. Moreover, the receptor-deficient mice showed larger long-term potentiation in the hippocampal CA1 region than control mice, without apparent changes in presynaptic or postsynaptic electrophysiological properties. These results show that the loss of the nociceptin receptor results in a gain-of-function mutation in both the memory process and the long-term potentiation mechanism in CA1, perhaps as a result of altered intracellular signal transduction systems in neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Meunier, J.-C. et al. Isolation and structure of the endogenous agonist of the opioid receptor-like ORL1 receptor. Nature 377, 532–535 (1995).
Reinscheid, R. K. et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794 (1995).
Henderson, G. & McKnight, A. T. The orphan opioid receptor and its endogenous ligand-nociceptin/orphanin FQ. Trends Pharmacol. Sci. 18, 293–300 (1997).
Nishi, M. et al. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 16, 1858–1864 (1997).
Morris, R. G. M. Spatial localization does not depend on the presence of local cues. Learn. Motiv. 12, 239–260 (1981).
Tsien, J. Z., Huerta, P. T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996).
Yamada, K. et al. The role of nitric oxide in dizocilpine-induced impairment of spontaneous alternation behavior in mice. J. Pharmacol. Exp. Ther. 276, 460–466 (1996).
Bliss, T. V. P. & Collingridge, G. L. Asynaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Knoflach, F., Reinscheid, R. K., Civelli, O. & Kemp, J. A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci. 16, 6657–6664 (1996).
Takahashi, T. & Momiyama, A. Different types of calcium channels mediate central synaptic transmission. Nature 366, 156–158 (1993).
Manabe, T., Wyllie, D. J. A., Perkel, D. J. & Nicoll, R. A. Modulation of synaptic transmission and long-term potentiation; effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J. Neurophysiol. 70, 1451–1459 (1993).
Ikeda, K. et al. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 45, 117–126 (1997).
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A. & Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature 307, 462–465 (1984).
Mayer, M. L., Westbrook, G. L. & Guthrie, P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature 309, 261–263 (1984).
Mamiya, T., Noda, Y., Nishi, M., Takeshima, H. & Nabeshima, T. Enhancement of spatial attention in nociceptin receptor-knockout mice. Brain Res. 783, 236–240 (1998).
Nabeshima, T. & Ichihara, K. Paradigms for the Study of Behavior, Vol. 14 (ed. Conn, P. M.) 217–229 (Academic, San Diego, (1993)).
Sandin, J., Georgieva, A. J., Schott, P. A., Ogren, S. O. & Terenius, L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 9, 194–197 (1997).
Yu, T. P., Fein, J., Phan, T., Evans, C. J. & Xie, C. W. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus 7, 88–94 (1997).
Madison, D. V. & Nicoll, R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature 299, 636–638 (1982).
Frey, U., Huang, Y.-Y. & Kandel, E. R. Effects of cAMP simulate a late phase of LTP in hippocampal CA1 neurons. Science 260, 1661–1664 (1993).
Blitzer, R. D., Wong, T., Nouranifar, R., Iyengar, R. & Landau, E. M. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 15, 1403–1414 (1995).
Thomas, M. J., Moody, T. D., Makhinson, M. & O'Dell, T. J. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron 17, 475–482 (1996).
Hu, G.-Y. et al. Protein kinase C injection into hippocampal pyramidal cells elicits features of long-term potentiation. Nature 328, 426–429 (1987).
Malinow, R., Schulman, H. & Tsien, R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866 (1989).
Lou, L.-G., Ma, L. L. & Pei, G. Nociceptin/orphanin FQ activates protein kinase C, and this effect is mediated through phospholipase C/Ca2+ pathway. Biochem. Biophys. Res. Commun. 240, 304–308 (1997).
Fukuda, K., Shoda, T., Morikawa, H., Kato, S. & Mori, K. Activation of mitogen-activated protein kinase by the nociceptin receptor expressed in Chinese hamster ovary cells. FEBS Lett. 412, 290–294 (1997).
Bajocchi, G., Feldman, S. H., Crystal, R. G. & Mastrangeli, A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vector. Nature Genet. 3, 229–234 (1993).
Houtani, T., Nishi, M., Takeshima, H., Nukada, T. & Sugimoto, T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem. Biophys. Res. Commun. 219, 714–719 (1996).
Acknowledgements
We thank A. Sakamoto and I. Dobashi for help maintaining the mutant mice. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Japan Private School Promotion Foundation, the Uehara Memorial Foundation, the TMFC and the Life Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manabe, T., Noda, Y., Mamiya, T. et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature 394, 577–581 (1998). https://doi.org/10.1038/29073
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/29073
This article is cited by
-
The NOP Receptor System in Neurological and Psychiatric Disorders: Discrepancies, Peculiarities and Clinical Progress in Developing Targeted Therapies
CNS Drugs (2021)
-
Transcriptomics of Gabra4 knockout mice reveals common NMDAR pathways underlying autism, memory, and epilepsy
Molecular Autism (2020)
-
A systematic review of the role of the nociceptin receptor system in stress, cognition, and reward: relevance to schizophrenia
Translational Psychiatry (2018)
-
Activation of Nociceptin/Orphanin FQ Peptide Receptors Disrupts Visual but Not Auditory Sensorimotor Gating in BALB/cByJ Mice: Comparison to Dopamine Receptor Agonists
Neuropsychopharmacology (2012)
-
Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64-6198 in rodent models of conflict anxiety and despair
Psychopharmacology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.