Abstract
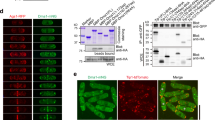
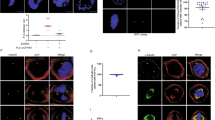
Here we show that p53 protein is physically associated with tubulin in vivo and in vitro, and that it localizes to cellular microtubules. Treatment with vincristine or paclitaxel before DNA-damage or before leptomycin B treatment reduces nuclear accumulation of p53 and expression of mdm2 and p21. Overexpression of dynamitin or microinjection of anti-dynein antibody before DNA damage abrogates nuclear accumulation of p53. Our results indicate that transport of p53 along microtubules is dynein-dependent. The first 25 amino acids of p53 contain the residues that are essential for binding to microtubules. We propose that functional microtubules and the dynein motor protein participate in transport of p53 and facilitate its accumulation in the nucleus after DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ko, L. J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Levine, A. J. p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997).
Vogelstein, B. & Kinzler, K. W. p53 function and dysfunction . Cell 70, 523–536 (1992).
Cross, S. M. et al. A p53-dependent mouse spindle checkpoint. Science 267, 1353–1356 (1995).
Fukasawa, K., Choi, T., Kuriyama, R., Rulong, S. & Vande, W. G. Abnormal centrosome amplification in the absence of p53. Science 271, 1744– 1747 (1996).
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51 , 6304–6311 (1991).
Lane, D. P. Cancer. p53, guardian of the genome. Nature 358, 15–16 (1992).
Prives, C. Signaling to p53: breaking the MDM2–p53 circuit. Cell 95, 5–8 (1998).
el-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W. & Vogelstein, B. A model for p53-induced apoptosis. Nature 389, 300–305 (1997).
Hollstein, M., Sidransky, D., Vogelstein, B. & Harris, C. C. p53 mutations in human cancers. Science 253, 49–53 (1991).
Levine, A. J., Momand, J. & Finlay, C. A. The p53 tumour suppressor gene. Nature 351, 453–456 (1991).
Momand, J., Zambetti, G. P., Olson, D. C., George, D. & Levine, A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation . Cell 69, 1237–1245 (1992).
Oliner, J. D. et al. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 362, 857– 860 (1993).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Moll, U. M. et al. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol. Cell. Biol. 16, 1126–1137 (1996).
Moll, U. M., Riou, G. & Levine, A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl Acad. Sci. USA 89, 7262–7266 (1992).
Schlamp, C. L., Poulsen, G. L., Nork, T. M. & Nickells, R. W. Nuclear exclusion of wild-type p53 in immortalized human retinoblastoma cells . J. Natl Cancer Inst. 89, 1530– 1536 (1997).
Bosari, S. et al. Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J. Natl Cancer Inst. 86, 681–687 (1994).
Sun, X. F. et al. Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet 340, 1369 –1373 (1992).
Freedman, D. A. & Levine, A. J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell Biol. 18, 7288– 7293 (1998).
Giannakakou, P. et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J. Biol. Chem. 272, 17118–17125 (1997).
Giannakakou, P. et al. Paclitaxel selects for mutant or pseudo-null p53 in drug resistance associated with tubulin mutations in human cancer. Oncogene 19, 3078–3085 (2000).
Hyman, A. A. & Karsenti, E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell 84, 401 –410 (1996).
Jordan, M. A. & Wilson, L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 10, 123–130 (1998).
Gundersen, G. G. & Cook, T. A. Microtubules and signal transduction. Curr. Opin. Cell Biol. 11, 81–94 (1999).
Stommel, J. M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking . EMBO J. 18, 1660–1672 (1999).
Goodson, H. V., Valetti, C. & Kreis, T. E. Motors and membrane traffic. Curr. Opin. Cell Biol. 9, 18–28 (1997).
Okada, Y. & Hirokawa, N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science 283, 1152–1157 (1999).
Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport . Science 279, 519–526 (1998).
Burkhardt, J. K., Echeverri, C. J., Nilsson, T. & Vallee, R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 (1997).
Klotzke, O., Etzrodt, D., Hohenberg, H., Bohn, W. & Deppert, W. Cytoplasmic retention of mutant tsp53 is dependent on an intermediate filament protein (vimentin) scaffold . Oncogene 16, 3423–3434 (1998).
Jimenez, G. S., Khan, S. H., Stommel, J. M. & Wahl, G. M. p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene 18, 7656–7665 (1999).
Maxwell, S. A. et al. Simian virus 40 large T antigen and p53 are microtubule-associated proteins in transformed cells. Cell Growth Differ. 2, 115–127 (1991).
Blagosklonny, M. V. et al. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer Res. 55, 4623–4626 (1995).
Kapeller, R., Toker, A., Cantley, L. C. & Carpenter, C. L. Phosphoinositide 3-kinase binds constitutively to alpha/beta-tubulin and binds to gamma-tubulin in response to insulin. J. Biol. Chem. 270, 25985–25991 (1995).
Magnelli, L. The old and the new in p53 functional regulation. Biochem. Mol. Med. 62, 3–10 (1997).
Chai, Y. L. et al. The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene 18, 263–268 (1999).
Ouchi, T., Monteiro, A. N., August, A., Aaronson, S. A. & Hanafusa, H. BRCA1 regulates p53-dependent gene expression. Proc. Natl Acad. Sci. USA 95, 2302–2306 (1998).
Hsu, L. C. & White, R. L. BRCA1 is associated with the centrosome during mitosis. Proc. Natl Acad. Sci. USA 95, 12983–12988 (1998).
Sackett, D. L., Knipling, L. & Wolff, J. Isolation of microtubule protein from mammalian brain frozen for extended periods of time. Protein Expr. Purif. 2, 390–393 (1991).
Takenaka, I., Morin, F., Seizinger, B. R. & Kley, N. Regulation of the sequence-specific DNA binding function of p53 by protein kinase C and protein phosphatases J. Biol. Chem. 270, 5405–5411 (1995).
Wolff, J., Sackett, D. L. & Knipling, L. Cation selective promotion of tubulin polymerization by alkali metal chlorides. Protein Sci. 5, 2020–2028 (1996).
Balczon, R., Varden, C. E. & Schroer, T. A. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell Motil. Cytoskeleton 42, 60–72 (1999).
Horton, R. M., Cai, Z. L., Ho, S. N. & Pease, L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction . Biotechniques 8, 528– 531 (1990).
Acknowledgements
We thank H. Gray and U. Roongta for help with expression and purification of His–p53 fusion protein. We are grateful to M. Clarke and C. Harris for providing full-length wild-type p53 and p53 (Δ342–end) plasmids, respectively. We also thank R. Vallee for providing the dynamitin plasmid, and L. Neckers for providing Leptomycin B. We are indebted to R. Klausner for insightful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence and requests for materials should be addressed to P.G.
Rights and permissions
About this article
Cite this article
Giannakakou, P., Sackett, D., Ward, Y. et al. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol 2, 709–717 (2000). https://doi.org/10.1038/35036335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35036335
This article is cited by
-
Doublecortin undergo nucleocytoplasmic transport via the RanGTPase signaling to promote glioma progression
Cell Communication and Signaling (2020)
-
P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease
Acta Neuropathologica Communications (2020)
-
β-Actin facilitates etoposide-induced p53 nuclear import
Journal of Biosciences (2020)
-
Interplay between HSF1 and p53 signaling pathways in cancer initiation and progression: non-oncogene and oncogene addiction
Cellular Oncology (2019)
-
Microenvironment dependent gene expression signatures in reprogrammed human colon normal and cancer cell lines
BMC Cancer (2018)