Abstract
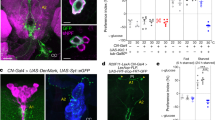
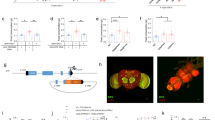
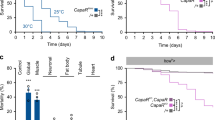
Antagonistic activities of glucagon and insulin control metabolism in mammals, and disruption of this balance underlies diabetes pathogenesis. Insulin-producing cells (IPCs) in the brain of insects such as Drosophila also regulate serum glucose1,2, but it remains unclear whether insulin is the sole hormonal regulator of glucose homeostasis and whether mechanisms of glucose-sensing and response in IPCs resemble those in pancreatic islets. Here we show, by targeted cell ablation, that Drosophila corpora cardiaca (CC) cells3,4,5 of the ring gland are also essential for larval glucose homeostasis. Unlike IPCs, CC cells express Drosophila cognates of sulphonylurea receptor (Sur) and potassium channel (Ir), proteins that comprise ATP-sensitive potassium channels regulating hormone secretion by islets and other mammalian glucose-sensing cells6,7,8. They also produce adipokinetic hormone, a polypeptide with glucagon-like functions. Glucose regulation by CC cells is impaired by exposure to sulphonylureas, drugs that target the Sur subunit. Furthermore, ubiquitous expression of an akh transgene reverses the effect of CC ablation on serum glucose. Thus, Drosophila CC cells are crucial regulators of glucose homeostasis and they use glucose-sensing and response mechanisms similar to islet cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rulifson, E. J., Kim, S. K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (2002)
Ikeya, T., Galic, M., Belawat, P., Nairz, K. & Hafen, E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12, 1293–1300 (2002)
Veelaert, D., Schoofs, L. & De Loof, A. Peptidergic control of the corpus cardiacum-corpora allata complex of locusts. Int. Rev. Cytol. 182, 249–302 (1998)
Van der Horst, D. J. Insect adipokinetic hormones: release and integration of flight energy metabolism. Comp. Biochem. Phys. B 136, 217–226 (2003)
Noyes, B. E., Katz, F. N. & Schaffer, M. H. Identification and expression of the Drosophila adipokinetic hormone gene. Mol. Cell. Endocrinol. 109, 133–141 (1995)
Aguilar-Bryan, L. et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 (1995)
Miki, T. et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nature Neurosci. 4, 507–512 (2001)
Seino, S. & Miki, T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 81, 133–176 (2003)
Staubli, F. et al. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl Acad. Sci. USA 99, 3446–3451 (2002)
McNabb, S. L. et al. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron 19, 813–823 (1997)
Lee, G. & Park, J. H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetichormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323 (2004)
Gelling, R. W. et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl Acad. Sci. USA 100, 1438–1443 (2003)
Brogiolo, W. et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213–221 (2001)
Nasonkin, I. et al. A novel sulfonylurea receptor family member expressed in the embryonic Drosophila dorsal vessel and tracheal system. J. Biol. Chem. 274, 29420–29425 (1999)
Döring, F., Wischmeyer, E., Kuhnlein, R. P., Jäckle, H. & Karschin, A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors Kir channel function. J. Biol. Chem. 277, 25554–25561 (2002)
Sturgess, N. C., Ashford, M. L., Cook, D. L. & Hales, C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet ii, 474–475 (1985)
Trube, G., Rorsman, P. & Ohno-Shosaku, T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflügers Arch. 407, 493–499 (1986)
Ostergard, T. et al. The insulin secretagogues glibenclamide and repaglinide do not influence growth hormone secretion in humans but stimulate glucagon secretion during profound insulin deficiency. J. Clin. Endocrinol. Metab. 89, 297–302 (2004)
Paradis, S., Sweeney, S. T. & Davis, G. W. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron 30, 737–747 (2001)
Göpel, S. O. et al. Regulation of glucagon release in mouse-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J. Physiol. (Lond.) 528, 509–520 (2000)
Høy, M. et al. Tolbutamide stimulates exocytosis of glucagon by inhibition of a mitochondrial-like ATP-sensitive K+ (KATP) conductance in rat pancreatic α-cells. J. Physiol. (Lond.) 527, 109–120 (2000)
Bokvist, K. et al. Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic α-cells. Pflügers Arch. 438, 428–436 (1999)
Yu, D., Baird, G. S., Tsien, R. Y. & Davis, R. L. Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. J. Neurosci. 23, 64–72 (2003)
Pannabecker, T. & Orchard, I. Regulation of adipokinetic hormone release from locust neuroendocrine tissue: participation of calcium and cyclic AMP. Brain Res. 423, 13–22 (1987)
Pannabecker, T. & Orchard, I. Ionic dependence of depolarization-mediated adipokinetic hormone release from the locust corpus cardiacum. Brain Res. 477, 38–47 (1989)
Passier, P., Vullings, H. G. B., Diederen, J. H. B. & Van der Horst, D. J. Trehalose inhibits the release of adipokinetic hormones from the corpus cardiacum in the African migratory locust, Locusta migratoria, at the level of the adipokinetic cells. J. Endocrinol. 153, 299–305 (1997)
Tomancak, P. et al. Berkeley Drosophila Genome Project (http://www.fruitfly.org/cgi-bin/ex/insitu.pl).
Orchard, I. & Loughton, B. G. Is octopamine a transmitter mediating hormone release in insects? J. Neurobiol. 12, 143–153 (1981)
Copenhaver, P. F. & Taghert, P. H. Origins of the insect enteric nervous system: differentiation of the enteric ganglia from a neurogenic epithelium. Development 113, 111–132 (1991)
DeVelasco, B., Shen, J., Go, S. & Hartenstein, V. Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass. Dev. Biol. (in the press)
Acknowledgements
We thank B. Tsai for Ir and Sur cloning and in situ hybridizations, X. Gu for akh cloning and constructions, G. McLean for fly husbandry, E. Bustamante for glucose assays, M. Fish for P-element-mediated transformations, P. Calvert for quantitative analysis of calcium transients, and R. Nusse, G. Barsh, M. Krasnow, S. DiNardo and members of the Rulifson and Kim laboratories for suggestions on the manuscript. E.R. was supported by a grant from the Juvenile Diabetes Research Foundation (JDRF). S.K. was supported by grants from the JDRF, the Pew Charitable Trusts and the Verto Institute. E.R. and S.K. contributed equally to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, S., Rulifson, E. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, 316–320 (2004). https://doi.org/10.1038/nature02897
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02897
This article is cited by
-
2D material graphene as a potential antidiabetic and nontoxic compound in Drosophila melanogaster
Applied Nanoscience (2024)
-
Gut AstA mediates sleep deprivation-induced energy wasting in Drosophila
Cell Discovery (2023)
-
Dietary cysteine drives body fat loss via FMRFamide signaling in Drosophila and mouse
Cell Research (2023)
-
No sugar, just protein please — says the fly
Nature Metabolism (2022)
-
The gut hormone Allatostatin C/Somatostatin regulates food intake and metabolic homeostasis under nutrient stress
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.