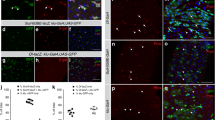
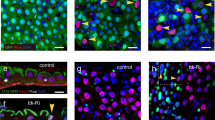
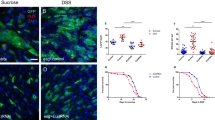
Abstract
Adult stem cells maintain organ systems throughout the course of life and facilitate repair after injury or disease1. A fundamental property of stem and progenitor cell division is the capacity to retain a proliferative state or generate differentiated daughter cells2; however, little is currently known about signals that regulate the balance between these processes. Here, we characterize a proliferating cellular compartment in the adult Drosophila midgut. Using genetic mosaic analysis we demonstrate that differentiated cells in the epithelium arise from a common lineage. Furthermore, we show that reduction of Notch signalling leads to an increase in the number of midgut progenitor cells, whereas activation of the Notch pathway leads to a decrease in proliferation. Thus, the midgut progenitor's default state is proliferation, which is inhibited through the Notch signalling pathway. The ability to identify, manipulate and genetically trace cell lineages in the midgut should lead to the discovery of additional genes that regulate stem and progenitor cell biology in the gastrointestinal tract.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Radtke, F. & Clevers, H. Self-renewal and cancer of the gut: Two sides of a coin. Science 307, 1904–1909 (2005)
Molofsky, A. V., Pardal, R. & Morrison, S. J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 16, 700–707 (2004)
Strasburger, M. Bau, Funktion und Variabilität des Darmtractus von Drosophila melanogaster Meigen. Zeit. f. wiss. Zool. 140, 539–649 (1932)
Wigglesworth, V. The Principles of Insect Physiology (Methuen & Co. Ltd, London, 1965)
Miller, A. in The Biology of Drosophila (ed. Demerec, M.) 420–442 (Hafner, New York, 1950)
Bozuck, A. N. DNA synthesis in the absence of somatic cell division associated with ageing in Drosophila subobscura. Exp. Geront. 7, 147–156 (1972)
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neural morphogenesis. Neuron 22, 451–461 (1999)
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993)
Furriols, M. & Bray, S. J. A model Notch response element detects Suppressor of Hairless dependent molecular switch. Curr. Biol. 11, 60–64 (2001)
Fuse, N., Hirose, S. & Hayashi, S. Diploidy of Drosophila imaginal cells is maintained by a transcriptional repressor encoded by escargot. Genes Dev. 8, 2270–2281 (1994)
Kiger, A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000)
McGuire, S. E. et al. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 (2003)
Presente, A., Shaw, S., Nye, J. S. & Andres, A. J. Transgene-mediated RNA interference defines a novel role for Notch in chemosensory startle behaviour. Genesis 34, 165–169 (2002)
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature advance online publication, 7 December 2005 (doi:10.1038/nature04333).
Torii, M. et al. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development 126, 443–456 (1999)
Li, L. & Vaessin, H. Pan-neural prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev. 14, 147–151 (2000)
Schonhoff, S. E., Giel-Moloney, M. & Leiter, A. B. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 145, 2639–2644 (2005)
Xie, T. & Spradling, A. in Stem Cell Biology (eds Marshak, D. R., Gardner, R. L. & Gottlieb, D.) 129–148 (Cold Spring Harbor Press, New York, 2001)
Kiger, A. & Fuller, M. in Stem Cell Biology (eds Marshak, D. R, Gardner, R. L. & Gottlieb, D.) 149–187 (Cold Spring Harbor Press, New York, 2001)
Acknowledgements
We thank GETDB and Bloomington stock centers for providing fly strains. C.A.M. would like to acknowledge colleagues in the Perrimon laboratory for many constructive discussions and criticisms; B. Mathey-Prevot for comments on the manuscript; K. Brückner for help in translating the seminal text by M. Strasburger from German; R. Binari for technical assistance; and S. Tang and C. Villata for help in screening. C.A.M. was supported by a NRSA fellowship and a grant from the Harvard Stem Cell Institute. N.P. is a HHMI investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure Legends
Text to accompany Supplementary Figures 1–4. (DOC 20 kb)
Supplementary Figure 1
Whole mount adult Drosophila gastrointestinal tract imaged in cross-section. (PDF 17 kb)
Supplementary Figure 2
esg+ cells define a population of midgut progenitors. (PDF 14 kb)
Supplementary Figure 3
Gal80ts provides control of transgene expression in the adult midgut. (PDF 16 kb)
Supplementary Figure 4
A model of the adult Drosophila midgut. (PDF 17 kb)
Rights and permissions
About this article
Cite this article
Micchelli, C., Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006). https://doi.org/10.1038/nature04371
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04371
This article is cited by
-
The emergence of circadian timekeeping in the intestine
Nature Communications (2024)
-
A single-cell atlas of Drosophila trachea reveals glycosylation-mediated Notch signaling in cell fate specification
Nature Communications (2024)
-
Drosophila activins adapt gut size to food intake and promote regenerative growth
Nature Communications (2024)
-
Cell-fate conversion of intestinal cells in adult Drosophila midgut by depleting a single transcription factor
Nature Communications (2024)
-
Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system
Nature Aging (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.