Abstract
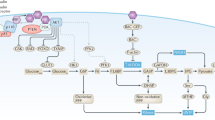
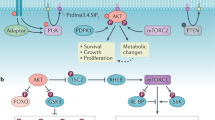
On activation by receptors, the ubiquitously expressed class IA isoforms (p110α and p110β) of phosphatidylinositol-3-OH kinase (PI(3)K) generate lipid second messengers, which initiate multiple signal transduction cascades1,2,3,4,5. Recent studies have demonstrated specific functions for p110α in growth factor and insulin signalling6,7,8. To probe for distinct functions of p110β, we constructed conditional knockout mice. Here we show that ablation of p110β in the livers of the resulting mice leads to impaired insulin sensitivity and glucose homeostasis, while having little effect on phosphorylation of Akt, suggesting the involvement of a kinase-independent role of p110β in insulin metabolic action. Using established mouse embryonic fibroblasts, we found that removal of p110β also had little effect on Akt phosphorylation in response to stimulation by insulin and epidermal growth factor, but resulted in retarded cell proliferation. Reconstitution of p110β-null cells with a wild-type or kinase-dead allele of p110β demonstrated that p110β possesses kinase-independent functions in regulating cell proliferation and trafficking. However, the kinase activity of p110β was required for G-protein-coupled receptor signalling triggered by lysophosphatidic acid and had a function in oncogenic transformation. Most strikingly, in an animal model of prostate tumour formation induced by Pten loss, ablation of p110β (also known as Pik3cb), but not that of p110α (also known as Pik3ca), impeded tumorigenesis with a concomitant diminution of Akt phosphorylation. Taken together, our findings demonstrate both kinase-dependent and kinase-independent functions for p110β, and strongly indicate the kinase-dependent functions of p110β as a promising target in cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
27 January 2016
A Correction to this paper has been published: https://doi.org/10.1038/nature16543
References
Vanhaesebroeck, B. & Waterfield, M. D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 253, 239–254 (1999)
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001)
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002)
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Rev. Genet. 7, 606–619 (2006)
Liu, Z. & Roberts, T. M. Human tumor mutants in the p110α subunit of PI3K. Cell Cycle 5, 675–677 (2006)
Foukas, L. C. et al. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441, 366–370 (2006)
Knight, Z. A. et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125, 733–747 (2006)
Zhao, J. J. et al. The p110α isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc. Natl Acad. Sci. USA 103, 16296–16300 (2006)
Bader, A. G., Kang, S., Zhao, L. & Vogt, P. K. Oncogenic PI3K deregulates transcription and translation. Nature Rev. Cancer 5, 921–929 (2005)
Vanhaesebroeck, B. et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602 (2001)
Bi, L., Okabe, I., Bernard, D. J., Wynshaw-Boris, A. & Nussbaum, R. L. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274, 10963–10968 (1999)
Bi, L., Okabe, I., Bernard, D. J. & Nussbaum, R. L. Early embryonic lethality in mice deficient in the p110β catalytic subunit of PI 3-kinase. Mamm. Genome 13, 169–172 (2002)
Brachmann, S. M., Ueki, K., Engelman, J. A., Kahn, R. C. & Cantley, L. C. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol. Cell. Biol. 25, 1596–1607 (2005)
Shaulian, E., Zauberman, A., Ginsberg, D. & Oren, M. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12, 5581–5592 (1992)
Hazeki, O. et al. Activation of PI 3-kinase by G protein βγ subunits. Life Sci. 62, 1555–1559 (1998)
Roche, S., Downward, J., Raynal, P. & Courtneidge, S. A. A function for phosphatidylinositol 3-kinase β (p85α-p110β) in fibroblasts during mitogenesis: requirement for insulin- and lysophosphatidic acid-mediated signal transduction. Mol. Cell. Biol. 18, 7119–7129 (1998)
Yart, A. et al. A function for phosphoinositide 3-kinase β lipid products in coupling βγ to Ras activation in response to lysophosphatidic acid. J. Biol. Chem. 277, 21167–21178 (2002)
Nobukuni, T. et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl Acad. Sci. USA 102, 14238–14243 (2005)
Shin, H. W. et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170, 607–618 (2005)
Daniels, T. R., Delgado, T., Rodriguez, J. A., Helguera, G. & Penichet, M. L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 121, 144–158 (2006)
Bellacosa, A. et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 64, 280–285 (1995)
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997)
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997)
Ringel, M. D. et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 61, 6105–6111 (2001)
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004)
Zhao, J. J. et al. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl Acad. Sci. USA 102, 18443–18448 (2005)
Bader, A. G., Kang, S. & Vogt, P. K. Cancer-specific mutations in PIK3CA are oncogenic in vivo . Proc. Natl Acad. Sci. USA 103, 1475–1479 (2006)
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005)
Lesche, R. et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32, 148–149 (2002)
Wu, X. et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 101, 61–69 (2001)
Taniguchi, C. M. et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metab. 3, 343–353 (2006)
Graner, E. et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5, 253–261 (2004)
Sever, S., Damke, H. & Schmid, S. L. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150, 1137–1148 (2000)
Emmert-Buck, M. R. et al. Laser capture microdissection. Science 274, 998–1001 (1996)
Acknowledgements
We thank C. D. Stiles and J. D. Iglehart for advice, and H. Wu for providing floxed PTEN mice. This work was supported by grants from the National Institutes of Health (M.L., T.M.R. and J.J.Z.,), the Department of Defense for Cancer Research (J.J.Z.), the V Foundation (J.J.Z.) and the Claudia Barr Program (J.J.Z.). In compliance with Harvard Medical School guidelines, we disclose the consulting relationships: Novartis Pharmaceuticals, Inc. (M.L., T.M.R. and J.J.Z.).
Author Contributions Z.L., S.Z. and S.L. generated the floxed p110β mouse. S.J. carried out mouse tumorigenesis studies. Z.L and S.Z. performed MEF studies. P.L. performed in vivo metabolic studies. L.Z. performed transferrin uptake assays. J.Z. assisted in focus formation and BrdU incorporation experiments. S.S. and M.L. performed and interpreted pathological analyses of mouse prostate tumors. T.M.R. and J.J.Z. supervised the research, interpreted the data and wrote the paper. S.J., Z.L., S.Z., P.L., L.Z., S.L. and M.L. participated in the writing of the paper.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-12 and Legends. The Supplementary Figures and Legends show additional data to support the kinase-dependent and -independent functions of PI3K-p110:β in cell growth, metabolism and tumorigenesis. (PDF 2415 kb)
Rights and permissions
About this article
Cite this article
Jia, S., Liu, Z., Zhang, S. et al. Essential roles of PI(3)K–p110β in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 (2008). https://doi.org/10.1038/nature07091
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07091
This article is cited by
-
Beyond PI3Ks: targeting phosphoinositide kinases in disease
Nature Reviews Drug Discovery (2023)
-
Antitumor activity of the PI3K δ-sparing inhibitor MEN1611 in PIK3CA mutated, trastuzumab-resistant HER2 + breast cancer
Breast Cancer Research and Treatment (2023)
-
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer
Molecular Cancer (2023)
-
Targeting signaling pathways in prostate cancer: mechanisms and clinical trials
Signal Transduction and Targeted Therapy (2022)
-
PTEN loss correlates with T cell exclusion across human cancers
BMC Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.