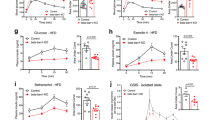
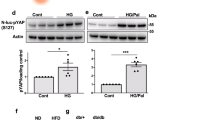
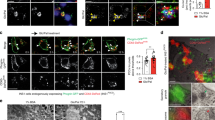
Abstract
Insulin resistance, a hallmark of type 2 diabetes, is a defect of insulin in stimulating insulin receptor signalling1,2, which has become one of the most serious public health threats. Upon stimulation by insulin, insulin receptor recruits and phosphorylates insulin receptor substrate proteins3, leading to activation of the phosphatidylinositol-3-OH kinase (PI(3)K)–Akt pathway. Activated Akt phosphorylates downstream kinases and transcription factors, thus mediating most of the metabolic actions of insulin4,5,6. β-arrestins mediate biological functions of G-protein-coupled receptors by linking activated receptors with distinct sets of accessory and effecter proteins, thereby determining the specificity, efficiency and capacity of signals7,8,9,10,11. Here we show that in diabetic mouse models, β-arrestin-2 is severely downregulated. Knockdown of β-arrestin-2 exacerbates insulin resistance, whereas administration of β-arrestin-2 restores insulin sensitivity in mice. Further investigation reveals that insulin stimulates the formation of a new β-arrestin-2 signal complex, in which β-arrestin-2 scaffolds Akt and Src to insulin receptor. Loss or dysfunction of β-arrestin-2 results in deficiency of this signal complex and disturbance of insulin signalling in vivo, thereby contributing to the development of insulin resistance and progression of type 2 diabetes. Our findings provide new insight into the molecular pathogenesis of insulin resistance, and implicate new preventive and therapeutic strategies against insulin resistance and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Matthaei, S., Stumvoll, M., Kellerer, M. & Haring, H. U. Pathophysiology and pharmacological treatment of insulin resistance. Endocr. Rev. 21, 585–618 (2000)
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signalling pathways: insights into insulin action. Nature Rev. Mol. Cell Biol. 7, 85–96 (2006)
Sun, X. J. et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352, 73–77 (1991)
Biddinger, S. B. & Kahn, C. R. From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 (2006)
Franke, T. F. et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 (1995)
Burgering, B. M. & Coffer, P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 (1995)
McDonald, P. H. et al. β-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 (2000)
Luttrell, L. M. et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl Acad. Sci. USA 98, 2449–2454 (2001)
Luttrell, L. M. et al. β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 (1999)
Beaulieu, J. M. et al. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273 (2005)
Beaulieu, J. M. et al. A β-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132, 125–136 (2008)
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005)
Chen, R. et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 276, 31858–31862 (2001)
Jiang, T. & Qiu, Y. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J. Biol. Chem. 278, 15789–15793 (2003)
Craxton, A., Jiang, A., Kurosaki, T. & Clark, E. A. Syk and Bruton’s tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 274, 30644–30650 (1999)
Wong, B. R. et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 4, 1041–1049 (1999)
Datta, K., Bellacosa, A., Chan, T. O. & Tsichlis, P. N. Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J. Biol. Chem. 271, 30835–30839 (1996)
DeWire, S. M., Ahn, S., Lefkowitz, R. J. & Shenoy, S. K. β-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 (2007)
Gao, H. et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol. Cell 14, 303–317 (2004)
Luan, B., Zhang, Z., Wu, Y., Kang, J. & Pei, G. β-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-κB activation. EMBO J. 24, 4237–4246 (2005)
Kang, J. et al. A nuclear function of β-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 123, 833–847 (2005)
Shi, Y. et al. Critical regulation of CD4+ T cell survival and autoimmunity by β-arrestin 1. Nature Immunol. 8, 817–824 (2007)
Netea, M. G. et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature Med. 12, 650–656 (2006)
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nature Med. 11, 191–198 (2005)
Shen, H. Q., Zhu, J. S. & Baron, A. D. Dose-response relationship of insulin to glucose fluxes in the awake and unrestrained mouse. Metabolism 48, 965–970 (1999)
Haluzik, M. M. et al. Improvement of insulin sensitivity after peroxisome proliferator-activated receptor-alpha agonist treatment is accompanied by paradoxical increase of circulating resistin levels. Endocrinology 147, 4517–4524 (2006)
Acknowledgements
We are grateful to R. J. Lefkowitz for providing us with β-arr2-KO mice. We thank J.-L. Guan for discussions and comments on the manuscripts. We thank all members of the laboratory for sharing reagents and advice. This research was supported by the Ministry of Science and Technology (2005CB522406, 2006CB943900, 2007CB947904, 2007CB947100, 2007CB948000 and 2009CB941100), National Natural Science Foundation of China (30621091, 30625014, 30623003, 30871285 and 90713047), Shanghai Municipal Commission for Science and Technology (07PJ14099 and 06DZ22032), Chinese Academy of Sciences (KSCX2-YW-R-56 and 2007KIP204).
Author Contributions This study was designed by B.L., J.Z. and G.P. The experiments were performed by B.L., B.D. and G.S. H.W. and W.J. contributed to the hyperinsulinaemic–euglycaemic clamp experiments. X.W. provided type 2 diabetes clinic samples. G.P. supervised the project. B.L. and J.Z. contributed to the writing of the paper. D.L. helped with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-7 with Legends (PDF 553 kb)
Rights and permissions
About this article
Cite this article
Luan, B., Zhao, J., Wu, H. et al. Deficiency of a β-arrestin-2 signal complex contributes to insulin resistance. Nature 457, 1146–1149 (2009). https://doi.org/10.1038/nature07617
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07617
This article is cited by
-
Effect of omega-3 fatty acids on glucose homeostasis: role of free fatty acid receptor 1
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Regulation of platelet-activating factor-induced interleukin-8 expression by protein tyrosine phosphatase 1B
Cell Communication and Signaling (2019)
-
A thorough analysis of diabetes research in China from 1995 to 2015: current scenario and future scope
Science China Life Sciences (2019)
-
Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat
Nature Communications (2018)
-
β-arrestin-2 is an essential regulator of pancreatic β-cell function under physiological and pathophysiological conditions
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.