Abstract
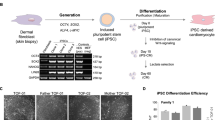
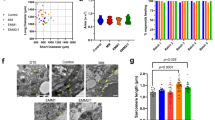
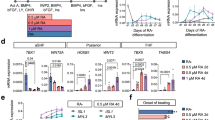
The generation of reprogrammed induced pluripotent stem cells (iPSCs) from patients with defined genetic disorders holds the promise of increased understanding of the aetiologies of complex diseases and may also facilitate the development of novel therapeutic interventions. We have generated iPSCs from patients with LEOPARD syndrome (an acronym formed from its main features; that is, lentigines, electrocardiographic abnormalities, ocular hypertelorism, pulmonary valve stenosis, abnormal genitalia, retardation of growth and deafness), an autosomal-dominant developmental disorder belonging to a relatively prevalent class of inherited RAS–mitogen-activated protein kinase signalling diseases, which also includes Noonan syndrome, with pleomorphic effects on several tissues and organ systems1,2. The patient-derived cells have a mutation in the PTPN11 gene, which encodes the SHP2 phosphatase. The iPSCs have been extensively characterized and produce multiple differentiated cell lineages. A major disease phenotype in patients with LEOPARD syndrome is hypertrophic cardiomyopathy. We show that in vitro-derived cardiomyocytes from LEOPARD syndrome iPSCs are larger, have a higher degree of sarcomeric organization and preferential localization of NFATC4 in the nucleus when compared with cardiomyocytes derived from human embryonic stem cells or wild-type iPSCs derived from a healthy brother of one of the LEOPARD syndrome patients. These features correlate with a potential hypertrophic state. We also provide molecular insights into signalling pathways that may promote the disease phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gorlin, R. J., Anderson, R. C. & Moller, J. H. The Leopard (multiple lentigines) syndrome revisited. Birth Defects Orig. Artic. Ser. 7, 110–115 (1971)
Sarkozy, A., Digilio, M. C. & Dallapiccola, B. Leopard syndrome. Orphanet J. Rare Dis. 3, 13 (2008)
Loh, M. L. et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 103, 2325–2331 (2004)
Tartaglia, M. et al. Genetic evidence for lineage-related and differentiation stage-related contribution of somatic PTPN11 mutations to leukemogenesis in childhood acute leukemia. Blood 104, 307–313 (2004)
Tartaglia, M. et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am. J. Hum. Genet. 78, 279–290 (2006)
Jopling, C., van Geemen, D. & den Hertog, J. Shp2 knockdown and Noonan/LEOPARD mutant Shp2-induced gastrulation defects. PLoS Genet. 3, e225 (2007)
Oishi, K. et al. Phosphatase-defective LEOPARD syndrome mutations in PTPN11 have gain-of-function effects during Drosophila development. Hum. Mol. Genet. 18, 193–201 (2008)
Lowry, W. E. et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl Acad. Sci. USA 105, 2883–2888 (2008)
Park, I. H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008)
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Ebert, A. D. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009)
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009)
Raya, A. et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460, 53–59 (2009)
Ye, Z. et al. Human induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114, 5473–5480 (2009)
Dimos, J. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008)
Laux, D., Kratz, C. & Sauerbrey, A. Common acute lymphoblastic leukemia in a girl with genetically confirmed LEOPARD syndrome. J. Pediatr. Hematol. Oncol. 30, 602–604 (2008)
Ucar, C., Calyskan, U., Martini, S. & Heinritz, W. Acute myelomonocytic leukemia in a boy with LEOPARD syndrome (PTPN11 gene mutation positive). J. Pediatr. Hematol. Oncol. 28, 123–125 (2006)
Mikkola, H. K., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516 (2003)
Wu, C. J. et al. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood 106, 3639–3645 (2005)
Fan, S. T. & Edgington, T. S. Coupling of the adhesive receptor CD11b/CD18 to functional enhancement of effector macrophage tissue factor response. J. Clin. Invest. 87, 50–57 (1991)
Aoki, H., Sadoshima, J. & Izumo, S. Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro . Nature Med. 6, 183–188 (2000)
Buitrago, M. et al. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nature Med. 11, 837–844 (2005)
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008)
Molkentin, J. D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63, 467–475 (2004)
Kontaridis, M. I., Swanson, K. D., David, F. S., Barford, D. & Neel, B. G. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J. Biol. Chem. 281, 6785–6792 (2006)
Edouard, T. et al. How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell. Mol. Life Sci. 64, 1585–1590 (2007)
Kennedy, M., D’Souza, S. L., Lynch-Kattman, M., Schwantz, S. & Keller, G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109, 2679–2687 (2007)
Grigoriadis, A. E. et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood 115, 2769–2776 (2010)
Dvorak, P. et al. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells 23, 1200–1211 (2005)
Freberg, C. T., Dahl, J. A., Timoskainen, S. & Collas, P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol. Biol. Cell 18, 1543–1553 (2007)
Carvajal-Vergara, X. et al. Multifunctional role of Erk5 in multiple myeloma. Blood 105, 4492–4499 (2005)
Acknowledgements
We thank T. James, X. Niu and D. York for their technical support and laboratory management, and B. MacArthur for support in microarray analysis. We also would like to thank K. Moore and her laboratory, and S. Mulero-Navarro from B.D.G.’s laboratory for their help, and V. Fuster and A. Bernad for their support. This research was funded by grants from the National Institutes of Health (NIH) to I.R.L (5R01GM078465), the Empire State Stem Cell Fund through New York State Department of Health (NYSTEM) C024410 to I.R.L. and C.S., C024176 (HESC-SRF) to I.R.L. and S.L.D., C024407 to B.D.G., American College of Cardiology/Pfizer Research Fellowship to E.D.A., and ERA-Net for research programmes on rare diseases 2009 to M.T. X.C.-V. is a recipient of a Postdoctoral Fellowship from the Ministerio de Ciencia e Innovacion/Instituto de Salud Carlos III, D.-F.L. is a New York Stem Cell Foundation Stanley and Fiona Druckenmiller Fellow and S.P. is a recipient of a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32).
Author information
Authors and Affiliations
Contributions
X.C.-V. (iPSC establishment, project planning, experimental work and preparation of manuscript); A.S., S.L.D., Y.-S.A., L.Y., A.D.K., E.D.A., D.-F.L., A.W., B.C., J.S. and S.P. (experimental work); R.R. and Y.G. (microarray analysis); N.C. and L.J.E. (karyotype analysis); K.D.L. and M.T. (obtaining of fibroblast samples from patients); C.S. (project planning, experimental work); B.D.G. and I.R.L. (project planning, preparation of manuscript).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with legends and Supplementary Table 1. (PDF 2117 kb)
Supplementary Movie 1
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line HES2. (MOV 5661 kb)
Supplementary Movie 2
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line L1-iPS6. (MOV 5718 kb)
Supplementary Movie 3
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line L1-iPS13. (MOV 2601 kb)
Supplementary Movie 4
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line L2-iPS6. (MOV 5762 kb)
Supplementary Movie 5
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line L2-iPS10. (MOV 6553 kb)
Supplementary Movie 6
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line L2iPS16. (MOV 15213 kb)
Supplementary Movie 7
This movie shows beating embryoid bodies at day 18 of cardiac differentiation: cell line S3-iPS4. (MOV 6356 kb)
Rights and permissions
About this article
Cite this article
Carvajal-Vergara, X., Sevilla, A., D’Souza, S. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010). https://doi.org/10.1038/nature09005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09005
This article is cited by
-
Sustainable and high-level microbial production of plant hemoglobin in Corynebacterium glutamicum
Biotechnology for Biofuels and Bioproducts (2023)
-
JAK2 as a surface marker for enrichment of human pluripotent stem cells-derived ventricular cardiomyocytes
Stem Cell Research & Therapy (2023)
-
Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism
Nature Cell Biology (2023)
-
An Assessment of the Therapeutic Landscape for the Treatment of Heart Disease in the RASopathies
Cardiovascular Drugs and Therapy (2023)
-
The Relevance of Programmed Cell Death to Spontaneous Defoliation in Sugarcane Leaf Sheaths
Sugar Tech (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.