Abstract
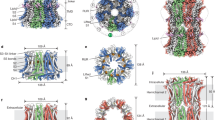
High-conductance voltage- and Ca2+-activated K+ channels function in many physiological processes that link cell membrane voltage and intracellular Ca2+ concentration, including neuronal electrical activity, skeletal and smooth muscle contraction, and hair cell tuning1,2,3,4,5,6,7,8. Like other voltage-dependent K+ channels, Ca2+-activated K+ channels open when the cell membrane depolarizes, but in contrast to other voltage-dependent K+ channels, they also open when intracellular Ca2+ concentrations rise. Channel opening by Ca2+ is made possible by a structure called the gating ring, which is located in the cytoplasm. Recent structural studies have defined the Ca2+-free, closed, conformation of the gating ring, but the Ca2+-bound, open, conformation is not yet known9. Here we present the Ca2+-bound conformation of the gating ring. This structure shows how one layer of the gating ring, in response to the binding of Ca2+, opens like the petals of a flower. The degree to which it opens explains how Ca2+ binding can open the transmembrane pore. These findings present a molecular basis for Ca2+ activation of K+ channels and suggest new possibilities for targeting the gating ring to treat conditions such as asthma and hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Robitaille, R., Garcia, M. L., Kaczorowski, G. J. & Charlton, M. P. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron 11, 645–655 (1993)
Fettiplace, R. & Fuchs, P. A. Mechanisms of hair cell tuning. Annu. Rev. Physiol. 61, 809–834 (1999)
Nelson, M. T. et al. Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 (1995)
Brenner, R. et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 (2000)
Petkov, G. V. et al. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. (Lond.) 537, 443–452 (2001)
Lee, U. S. & Cui, J. BK channel activation: structural and functional insights. Trends Neurosci. 33, 415–423 (2010)
Kaczorowski, G. J., Knaus, H. G., Leonard, R. J., McManus, O. B. & Garcia, M. L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. 28, 255–267 (1996)
Salkoff, L., Butler, A., Ferreira, G., Santi, C. & Wei, A. High-conductance potassium channels of the SLO family. Nature Rev. Neurosci. 7, 921–931 (2006)
Wu, Y., Yang, Y., Ye, S. & Jiang, Y. Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature 466, 393–397 (2010)
Jiang, Y., Pico, A., Cadene, M., Chait, B. T. & MacKinnon, R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron 29, 593–601 (2001)
Albright, R. A., Ibar, J. L., Kim, C. U., Gruner, S. M. & Morais-Cabral, J. H. The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell 126, 1147–1159 (2006)
Ye, S., Li, Y., Chen, L. & Jiang, Y. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell 126, 1161–1173 (2006)
Bakker, E. P., Booth, I. R., Dinnbier, U., Epstein, W. & Gajewska, A. Evidence for multiple K+ export systems in Escherichia coli . J. Bacteriol. 169, 3743–3749 (1987)
Roosild, T. P., Miller, S., Booth, I. R. & Choe, S. A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell 109, 781–791 (2002)
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002)
Bellamacina, C. R. The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins. FASEB J. 10, 1257–1269 (1996)
Dong, J., Shi, N., Berke, I., Chen, L. & Jiang, Y. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J. Biol. Chem. 280, 41716–41724 (2005)
Yuan, P., Leonetti, M. D., Pico, A. R., Hsiung, Y. & MacKinnon, R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science 329, 182–186 (2010)
Pico, A. R. RCK Domain Model of Calcium Activation in BK Channels 43–46. PhD thesis, Rockefeller Univ. (2003)
Niu, X., Qian, X. & Magleby, K. L. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron 42, 745–756 (2004)
Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001)
Wilkens, C. M. & Aldrich, R. W. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128, 347–364 (2006)
Li, W. & Aldrich, R. W. State-dependent block of BK channels by synthesized shaker ball peptides. J. Gen. Physiol. 128, 423–441 (2006)
Zhou, Y., Xia, X. M. & Lingle, C. J. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl Acad. Sci. USA 108, 12161–12166 (2011)
Tang, Q. Y., Zeng, X. H. & Lingle, C. J. Closed-channel block of BK potassium channels by bbTBA requires partial activation. J. Gen. Physiol. 134, 409–436 (2009)
Li, W. & Aldrich, R. W. Unique inner pore properties of BK channels revealed by quaternary ammonium block. J. Gen. Physiol. 124, 43–57 (2004)
Wu, R. S. & Marx, S. O. The BK potassium channel in the vascular smooth muscle and kidney: α- and β-subunits. Kidney Int. 78, 963–974 (2010)
Ghatta, S., Nimmagadda, D., Xu, X. & O’Rourke, S. T. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol. Ther. 110, 103–116 (2006)
Miura, M., Belvisi, M. G., Stretton, C. D., Yacoub, M. H. & Barnes, P. J. Role of potassium channels in bronchodilator responses in human airways. Am. Rev. Respir. Dis. 146, 132–136 (1992)
Jones, T. R., Charette, L., Garcia, M. L. & Kaczorowski, G. J. Interaction of iberiotoxin with β-adrenoceptor agonists and sodium nitroprusside on guinea pig trachea. J. Appl. Physiol. 74, 1879–1884 (1993)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Strong, M. et al. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis . Proc. Natl Acad. Sci. USA 103, 8060–8065 (2006)
Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. A graphical user interface to the CCP4 program suite. Acta Crystallogr. D 59, 1131–1137 (2003)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Brunger, A. T. Version 1.2 of the crystallography and NMR system. Nature Protocols 2, 2728–2733 (2007)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Echols, N., Milburn, D. & Gerstein, M. MolMovDB: analysis and visualization of conformational change and structural flexibility. Nucleic Acids Res. 31, 478–482 (2003)
Liman, E. R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992)
Acknowledgements
We thank staff members at NSLS X29, Brookhaven National Laboratory, for beamline assistance, and members of the MacKinnon laboratory for discussion. We thank P. Hoff and members of the Gadsby laboratory for help with oocyte preparation. R.M. is an investigator in the Howard Hughes Medical Institute. The research is supported by the American Asthma Foundation grant 07-0127.
Author information
Authors and Affiliations
Contributions
P.Y. purified and crystallized the protein, collected the X-ray diffraction data, determined the structure and conducted electrophysiology recordings. M.D.L. aided in initial crystallization and electrophysiology experiments. Y.H. provided assistance with protein expression. P.Y. and R.M. designed the research and analysed data. P.Y., M.D.L. and R.M. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Table 1 and Supplementary Figures 1-5 with legends. (PDF 3949 kb)
Supplementary Movie 1
This movie shows a morph between the Ca2+-free, closed and Ca2+-bound, open conformations of the BK channel gating ring (RCK1 in blue and RCK2 in red). At 20 seconds, a close-up view at the assembly interface around the Ca2+ bowl is started. At 30 seconds, the pore domain is shown on top of the gating ring. The modeling of the closed and the open conformations of the pore and the gating ring is described in the paper. The N-terminal residues Lys 343 from the RCK1 domains and the last residues from the inner helices are shown as black spheres. The linkers connecting the inner helices to the gating ring are illustrated as dashed lines colored in black. (MPG 16218 kb)
Supplementary Movie 2
This movie shows a morph between the closed (PDB 2FY8) and the open (PDB 1LNQ) conformations of the MthK channel gating ring. (MPG 3686 kb)
Rights and permissions
About this article
Cite this article
Yuan, P., Leonetti, M., Hsiung, Y. et al. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature 481, 94–97 (2012). https://doi.org/10.1038/nature10670
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10670
This article is cited by
-
Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM
Nature Communications (2021)
-
Purification and initial characterization of Plasmodium falciparum K+ channels, PfKch1 and PfKch2 produced in Saccharomyces cerevisiae
Microbial Cell Factories (2020)
-
Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations
Nature Communications (2019)
-
Molecular determinants of Ca2+ sensitivity at the intersubunit interface of the BK channel gating ring
Scientific Reports (2018)
-
Structural basis for gating the high-conductance Ca2+-activated K+ channel
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.