Abstract
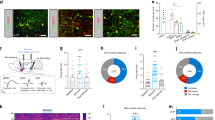
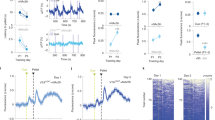
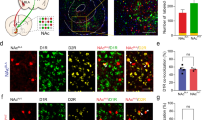
The ventral tegmental area (VTA) and nucleus accumbens (NAc) are essential for learning about environmental stimuli associated with motivationally relevant outcomes. The task of signalling such events, both rewarding and aversive, from the VTA to the NAc has largely been ascribed to dopamine neurons1,2,3. The VTA also contains GABA (γ-aminobutyric acid)-releasing neurons, which provide local inhibition4,5 and also project to the NAc6,7. However, the cellular targets and functional importance of this long-range inhibitory projection have not been ascertained. Here we show that GABA-releasing neurons of the VTA that project to the NAc (VTA GABA projection neurons) inhibit accumbal cholinergic interneurons (CINs) to enhance stimulus–outcome learning. Combining optogenetics with structural imaging and electrophysiology, we found that VTA GABA projection neurons selectively target NAc CINs, forming multiple symmetrical synaptic contacts that generated inhibitory postsynaptic currents. This is remarkable considering that CINs represent a very small population of all accumbal neurons, and provide the primary source of cholinergic tone in the NAc. Brief activation of this projection was sufficient to halt the spontaneous activity of NAc CINs, resembling the pause recorded in animals learning stimulus–outcome associations8,9,10,11,12. Indeed, we found that forcing CINs to pause in behaving mice enhanced discrimination of a motivationally important stimulus that had been associated with an aversive outcome. Our results demonstrate that VTA GABA projection neurons, through their selective targeting of accumbal CINs, provide a novel route through which the VTA communicates saliency to the NAc. VTA GABA projection neurons thus emerge as orchestrators of dopaminergic and cholinergic modulation in the NAc.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Everitt, B. J. et al. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. NY Acad. Sci. 877, 412–438 (1999)
Mirenowicz, J. & Schultz, W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379, 449–451 (1996)
Robinson, T. E. & Berridge, K. C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 18, 247–291 (1993)
Tan, K. R. et al. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183 (2012)
van Zessen, R., Phillips, J. L., Budygin, E. A. & Stuber, G. D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012)
Van Bockstaele, E. J. & Pickel, V. M. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 682, 215–221 (1995)
Margolis, E. B. et al. κ opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl Acad. Sci. USA 103, 2938–2942 (2006)
Kimura, M., Rajkowski, J. & Evarts, E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl Acad. Sci. USA 81, 4998–5001 (1984)
Aosaki, T. et al. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. 14, 3969–3984 (1994)
Goldberg, J. A. & Reynolds, J. N. J. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience 198, 27–43 (2011)
Apicella, P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 30, 299–306 (2007)
Joshua, M., Adler, A., Mitelman, R., Vaadia, E. & Bergman, H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684 (2008)
Kätzel, D., Zemelman, B. V., Buetfering, C., Wölfel, M. & Miesenböck, G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nature Neurosci. 14, 100–107 (2011)
English, D. F. et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nature Neurosci. 15, 123–130 (2012)
Ding, J. B., Guzman, J. N., Peterson, J. D., Goldberg, J. A. & Surmeier, D. J. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307 (2010)
Rymar, V. V., Sasseville, R., Luk, K. C. & Sadikot, A. F. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J. Comp. Neurol. 469, 325–339 (2004)
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012)
Inokawa, H., Yamada, H., Matsumoto, N., Muranishi, M. & Kimura, M. Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience 168, 395–404 (2010)
Apicella, P., Ravel, S., Deffains, M. & Legallet, E. The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance. J. Neurosci. 31, 1507–1515 (2011)
Matsumoto, N., Minamimoto, T., Graybiel, A. M. & Kimura, M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol. 85, 960–976 (2001)
Schulz, J. M., Oswald, M. J. & Reynolds, J. N. J. Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons. J. Neurosci. 31, 11133–11143 (2011)
Ligorio, M., Descarries, L. & Warren, R. A. Cholinergic innervation and thalamic input in rat nucleus accumbens. J. Chem. Neuroanat. 37, 33–45 (2009)
Cragg, S. J. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 29, 125–131 (2006)
Threlfell, S. et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012)
Cachope, R. et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41 (2012)
Pratt, W. E., Spencer, R. C. & Kelley, A. E. Muscarinic receptor antagonism of the nucleus accumbens core causes avoidance to flavor and spatial cues. Behav. Neurosci. 121, 1215–1223 (2007)
Hikida, T. et al. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc. Natl Acad. Sci. USA 98, 13351–13354 (2001)
Witten, I. B. et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681 (2010)
Nugent, F. S., Penick, E. C. & Kauer, J. A. Opioids block long-term potentiation of inhibitory synapses. Nature 446, 1086–1090 (2007)
Padgett, C. L. et al. Methamphetamine-evoked depression of GABAB receptor signaling in GABA neurons of the VTA. Neuron 73, 978–989 (2012)
Acknowledgements
We thank the members of the Lüscher laboratory for discussion and comments on the manuscript. This work was funded by a grant of the Swiss National Science Foundation to C.L. and the National Center of Competence in Research (NCCR) ‘SYNAPSY – The Synaptic Bases of Mental Diseases’ of the Swiss National Science Foundation. fTomPVALBcre mice were provided by A. Holtmaat.
Author information
Authors and Affiliations
Contributions
M.T.C.B., K.R.T. and E.C.O’C. performed the in vitro electrophysiological recordings. M.T.C.B. and K.R.T. performed the in vivo electrophysiological recordings. M.T.C.B. performed the fluorescence immunohistochemical experiments and confocal microscopy. I.N. and D.M. conceived and performed the light and electron microscopic experiments. E.C.O’C. performed the behavioural experiments. C.L., M.T.C.B., K.R.T. and E.C.O’C. designed the study, and C.L. wrote the manuscript with the help of M.T.C.B., K.R.T. and E.C.O’C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, additional references and Supplementary Figures 1-7. (PDF 1934 kb)
Rights and permissions
About this article
Cite this article
Brown, M., Tan, K., O’Connor, E. et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012). https://doi.org/10.1038/nature11657
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11657
This article is cited by
-
Updating the striatal–pallidal wiring diagram
Nature Neuroscience (2024)
-
Atypical antipsychotics antagonize GABAA receptors in the ventral tegmental area GABA neurons to relieve psychotic behaviors
Molecular Psychiatry (2023)
-
Gestational ethanol exposure impairs motor skills in female mice through dysregulated striatal dopamine and acetylcholine function
Neuropsychopharmacology (2023)
-
Drug reinforcement impairs cognitive flexibility by inhibiting striatal cholinergic neurons
Nature Communications (2023)
-
Role of α6-Nicotinic Receptors in Alcohol-Induced GABAergic Synaptic Transmission and Plasticity to Cholinergic Interneurons in the Nucleus Accumbens
Molecular Neurobiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.