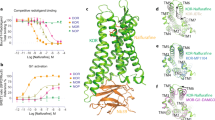
Abstract
Opioids represent widely prescribed and abused medications, although their signal transduction mechanisms are not well understood. Here we present the 1.8 Å high-resolution crystal structure of the human δ-opioid receptor (δ-OR), revealing the presence and fundamental role of a sodium ion in mediating allosteric control of receptor functional selectivity and constitutive activity. The distinctive δ-OR sodium ion site architecture is centrally located in a polar interaction network in the seven-transmembrane bundle core, with the sodium ion stabilizing a reduced agonist affinity state, and thereby modulating signal transduction. Site-directed mutagenesis and functional studies reveal that changing the allosteric sodium site residue Asn 131 to an alanine or a valine augments constitutive β-arrestin-mediated signalling. Asp95Ala, Asn310Ala and Asn314Ala mutations transform classical δ-opioid antagonists such as naltrindole into potent β-arrestin-biased agonists. The data establish the molecular basis for allosteric sodium ion control in opioid signalling, revealing that sodium-coordinating residues act as ‘efficacy switches’ at a prototypic G-protein-coupled receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pasternak, G. W. Opioids and their receptors: are we there yet? Neuropharmacology 76, 198–203 (2014)
Katritch, V., Cherezov, V. & Stevens, R. C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 (2013)
Wootten, D., Christopoulos, A. & Sexton, P. M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nature Rev. Drug Discov. 12, 630–644 (2013)
Rosenbaum, D. M., Rasmussen, S. G. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009)
Pert, C. B., Pasternak, G. & Snyder, S. H. Opiate agonists and antagonists discriminated by receptor binding in brain. Science 182, 1359–1361 (1973)
Cooper, D. M., Londos, C., Gill, D. L. & Rodbell, M. Opiate receptor-mediated inhibition of adenylate cyclase in rat striatal plasma membranes. J. Neurochem. 38, 1164–1167 (1982)
Portoghese, P. S., Sultana, M., Nagase, H. & Takemori, A. E. Application of the message-address concept in the design of highly potent and selective non-peptide δ opioid receptor antagonists. J. Med. Chem. 31, 281–282 (1988)
Granier, S. et al. Structure of the δ-opioid receptor bound to naltrindole. Nature 485, 400–404 (2012)
Ballesteros, J. A. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995)
Doré, A. S. et al. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011)
Park, J. H., Scheerer, P., Hofmann, K. P., Choe, H. W. & Ernst, O. P. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454, 183–187 (2008)
Thompson, A. A. et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485, 395–399 (2012)
Bonner, G., Meng, F. & Akil, H. Selectivity of μ-opioid receptor determined by interfacial residues near third extracellular loop. Eur. J. Pharmacol. 403, 37–44 (2000)
Filizola, M. & Devi, L. A. Grand opening of structure-guided design for novel opioids. Trends Pharmacol. Sci. 34, 6–12 (2013)
Burford, N. T. et al. Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor. Proc. Natl Acad. Sci. USA 110, 10830–10835 (2013)
Liu, W. et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337, 232–236 (2012)
Audet, M. & Bouvier, M. Restructuring G-protein-coupled receptor activation. Cell 151, 14–23 (2012)
Xu, F. et al. Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 (2011)
Selley, D. E., Cao, C. C., Liu, Q. & Childers, S. R. Effects of sodium on agonist efficacy for G-protein activation in μ-opioid receptor-transfected CHO cells and rat thalamus. Br. J. Pharmacol. 130, 987–996 (2000)
Yabaluri, N. & Medzihradsky, F. Regulation of μ-opioid receptor in neural cells by extracellular sodium. J. Neurochem. 68, 1053–1061 (1997)
Horstman, D. A. et al. An aspartate conserved among G-protein receptors confers allosteric regulation of alpha 2-adrenergic receptors by sodium. J. Biol. Chem. 265, 21590–21595 (1990)
Costa, T., Lang, J., Gless, C. & Herz, A. Spontaneous association between opioid receptors and GTP-binding regulatory proteins in native membranes: specific regulation by antagonists and sodium ions. Mol. Pharmacol. 37, 383–394 (1990)
Gao, Z. G. & IJzerman, A. P. Allosteric modulation of A(2A) adenosine receptors by amiloride analogues and sodium ions. Biochem. Pharmacol. 60, 669–676 (2000)
Neve, K. A. Regulation of dopamine D2 receptors by sodium and pH. Mol. Pharmacol. 39, 570–578 (1991)
Christopoulos, A. & Kenakin, T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54, 323–374 (2002)
Childers, S. R., Fleming, L. M., Selley, D. E., McNutt, R. W. & Chang, K. J. BW373U86: a nonpeptidic delta-opioid agonist with novel receptor-G protein-mediated actions in rat brain membranes and neuroblastoma cells. Mol. Pharmacol. 44, 827–834 (1993)
Holst, B. et al. Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J. Biol. Chem. 282, 15799–15811 (2007)
Steen, A. et al. Biased and constitutive signaling in the CC-chemokine receptor CCR5 by manipulating the interface between transmembrane helices 6 and 7. J. Biol. Chem. 288, 12511–12521 (2013)
Szekeres, P. G. & Traynor, J. R. Delta opioid modulation of the binding of guanosine-5′-O-(3-[35S]thio)triphosphate to NG108–15 cell membranes: characterization of agonist and inverse agonist effects. J. Pharmacol. Exp. Ther. 283, 1276–1284 (1997)
Liu, J. G. & Prather, P. L. Chronic agonist treatment converts antagonists into inverse agonists at δ-opioid receptors. J. Pharmacol. Exp. Ther. 302, 1070–1079 (2002)
Chu, R. et al. Redesign of a four-helix bundle protein by phage display coupled with proteolysis and structural characterization by NMR and X-ray crystallography. J. Mol. Biol. 323, 253–262 (2002)
Heckman, K. L. & Pease, L. R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nature Protocols 2, 924–932 (2007)
Wu, B. et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071 (2010)
Wacker, D. et al. Conserved binding mode of human β2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J. Am. Chem. Soc. 132, 11443–11445 (2010)
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nature Protocols 4, 706–731 (2009)
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y. F. & Caffrey, M. A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallogr. D 60, 1795–1807 (2004)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot . Acta Crystallogr. D 66, 486–501 (2010)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl Acad. Sci. USA 105, 64–69 (2008)
Allen, J. A. et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl Acad. Sci. USA 108, 18488–18493 (2011)
Carlsson, J. et al. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nature Chem. Biol. 7, 769–778 (2011)
Wacker, D. et al. Structural features for functional selectivity at serotonin receptors. Science 340, 615–619 (2013)
Besnard, J. et al. Automated design of ligands to polypharmacological profiles. Nature 492, 215–220 (2012)
Acknowledgements
This work was supported by the National Institutes of Health Common Fund grant P50 GM073197 for technology development (V.C. and R.C.S.), PSI:Biology grant U54 GM094618 for biological studies and structure production (target GPCR-39) (V.K., V.C. and R.C.S.), R01 DA017204 and the NIMH Psychoactive Drug Screening Program (P.G., X.-P.H., B.L.R.) and the Michael Hooker Chair for Protein Therapeutics and Translational Proteomics to B.L.R. We thank J. Velasquez for help with molecular biology, T. Trinh and M. Chu for help with baculovirus expression, G.W. Han for help with structure analysis and quality control review, E. Abola for help with sodium site analysis, A. Walker for assistance with manuscript preparation and J. Smith, R. Fischetti and N. Sanishvili for assistance in development and use of the minibeam and beamtime at beamline 23-ID at the Advanced Photon Source, which is supported by National Cancer Institute grant Y1-CO-1020 and National Institute of General Medical Sciences grant Y1-GM-1104.
Author information
Authors and Affiliations
Contributions
G.F. designed, optimized and purified δ-OR receptor constructs for structural studies, crystallized the receptor in LCP, collected and processed diffraction data, determined the structure, analysed the data and wrote the paper. P.M.G. performed mutagenesis and signalling studies, analysed the data and wrote the paper. X.-P.H. performed ligand binding and signalling studies, analysed the data and wrote the paper. V.K. analysed the data and wrote the paper. A.A.T. designed and cloned initial δ-OR constructs. V.C. analysed the data and wrote the paper. B.L.R. supervised the pharmacology and mutagenesis studies, analysed the data and wrote the paper. R.C.S. was responsible for the overall project strategy and management, analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Interactions in the orthosteric ligand-binding site of BRIL–δOR(ΔN/ΔC)–naltrindole.
a, ‘Close up’ view of the hydrogen-bond interaction network (yellow dotted lines) between the ligand naltrindole (orange sticks) and receptor (green cartoon; side chains shown as sticks) showing water (red spheres)-mediated interactions. b, Overview of water-mediated interactions (grey dotted lines) in the orthosteric pocket of BRIL–δOR(ΔN/ΔC)–naltrindole. c, mFo – DFc ‘omit’ electron density map (blue mesh) around naltrindole (orange sticks) after simulated annealing in PHENIX41 (5,000 K; contoured at 3σ); all water molecules surrounding the ligand in the orthosteric site are shown (red spheres). d, Surface representation of the BRIL–δOR(ΔN/ΔC)–naltrindole (green surface) showing the position of naltrindole (orange sticks) and the water cluster (red spheres) in the orthosteric site.
Extended Data Figure 2 Superposition of M. musculus and human δ-OR receptor structures.
a, Comparison of the human BRIL–δOR(ΔN/ΔC)–naltrindole structure (green) with the previously determined 3.4 Å crystal structure of the M. musculus δOR-T4L (magenta; PDB 4EJ4). The ligand naltrindole in the BRIL–δOR(ΔN/ΔC)–naltrindole structure is shown as orange spheres and is omitted in the 4EJ4 structure. Water molecules and the sodium ion in the allosteric site of the BRIL–δOR(ΔN/ΔC)–naltrindole structure are shown as red and blue spheres, respectively. The grey box represents the approximate regions of the receptor 7TM domain embedded in the lipidic bilayer; yellow sticks represent lipids. ICL3 fusion protein in 4EJ4 (T4L) and N-terminal BRIL fusion protein in BRIL–δOR(ΔN/ΔC)–naltrindole structures are omitted. b, ECL3 conformation of human δ-OR and comparison with mouse δOR–T4L ECL3 structure. BRIL–δOR(ΔN/ΔC)–naltrindole is shown as green cartoon and side chains are depicted as green sticks. The ligand naltrindole is represented by orange carbon sticks and transparent spheres (BRIL–δOR(ΔN/ΔC)–naltrindole structure) and magenta carbon sticks (4EJ4 structure).
Extended Data Figure 3 Conservation of the allosteric pocket in class A GPCRs harbouring a sodium ion and a water cluster.
a, Structural comparison of the sodium pocket between BRIL–δOR(ΔN/ΔC)–naltrindole structure (green cartoon and carbon atoms) and sodium-bound 1.8 Å resolution structure of A2AAR (PDB 4EIY; yellow cartoon and carbons). Water molecules of the pocket in the δ-OR structure are shown by red transparent spheres and smaller solid spheres, whereas water molecules in the A2AAR structure pocket are shown with small yellow solid spheres only. Sodium ions in both structures are shown with blue transparent spheres, with red dotted lines from the Na+ ion to the five coordinating oxygen atoms shown in the δ-OR structure. Part of the ligand naltrindole is shown as thick sticks with orange carbons. The comparison reveals identical side chains and similar conformations in 15 residue positions of the pocket. The important exception is the 3.35 position that in δ-OR features a polar Asn 1313.35 side chain coordinating the Na+ ion, whereas in A2AAR the Leu 873.35 side chain is oriented towards the lipidic membrane. b, Sequence comparison of the allosteric sodium pocket residues in currently available crystal structures of class A GPCRs. Asterisk marks receptors with high-resolution crystallographic evidence for sodium ions coordinated by the D2.50 side chain. Note that rhodopsin (OPSD) lacks polar residues in key positions 3.39, 7.45 and 7.46, which sets it apart from other class A receptors.
Extended Data Figure 4 Electron density maps around the δ-OR sodium-binding site.
a, 2mFo – DFc electron density map (grey mesh) of receptor residues (cyan sticks) around the sodium ion (blue sphere)-binding site contoured at 1σ. Black dashed lines represent interactions between the sodium ion and the atoms in direct contact with the receptor residues or water molecules in the first hydration shell of the ion. Yellow dashed lines show the hydrogen-bond network of water molecules and receptor residues. These polar interactions in the core of the 7TM bundle of the receptor establish an axis of connectivity between the orthosteric binding site, allosteric sodium site and residues in the intracellular side of the receptor. b, mFo – DFc ‘omit’ electron density map of sodium ion and coordinating water molecules after simulated annealing in PHENIX41 (5,000 K; contoured at 3σ).
Extended Data Figure 5 Functional characterization of wild-type δ-OR and the engineered BRIL–δOR(ΔN/ΔC) construct used for crystallization.
a, b, G-protein signalling characterization for wild-type (a) and BRIL–δOR(ΔN/ΔC) (b) is shown. Allosteric inhibitory effect of sodium on DADLE binding to 3H-naltrindole-labelled wild-type δ-OR expressed in HEK293 cells (positive control) (c) and the BRIL–δOR(ΔN/ΔC) construct used for crystallization and expressed in Sf9 cells (d). Ligand-binding data (d) were analysed using the allosteric model, and the results are shown in Table 1. Data represent the mean of four independent experiments each in quadruplicate.
Extended Data Figure 6 Effect of different cations on the binding of DADLE to BRIL–δOR(ΔN/ΔC).
a, b, The effects of different cations on both the total number of binding sites (relative Bmax in a) and affinity (pKd in b) of 3H-DADLE at the crystallized δ-OR construct expressed in Sf9 cells. 3H-DADLE saturation binding assays were conducted with control binding buffer (50 mM Tris HCl, pH 7.40) in the absence (control) and presence of 100 mM of the indicated chloride salts. Results were analysed in Prism to obtain Bmax and Kd values. The Bmax values were then normalized to control (100%) in each assay. Values represent mean ± s.e.m. from three independent assays, each in triplicate. *P < 0.05, one-way analysis of variance (ANOVA).
Extended Data Figure 7 Effect of sodium site mutations on basal Gαi and β-arrestin signalling.
HEK293 cells were transfected with 10 μg of receptor DNA and 10 μg of GloSensor per 15-cm dish and wild-type and mutant δ-ORs were assayed for Gαi signalling as described in Methods. a, Concentration-responses of wild-type-δ-OR-mediated Gαi signalling induced by the agonists BW373U86 and DADLE, the antagonist† naltrindole, and the inverse agonist ICI-174-864. b, Concentration-responses of δ-OR wild-type- and mutant-mediated Gαi signalling induced by the inverse agonist ICI-174-864. c, d, Cells were treated with 100 ng ml−1 pertussis toxin (PTX) for 12 h, depleting all Gαi constitutive activity. c, Non-normalized basal cAMP level of the δ-OR wild type and mutants in the presence or absence of PTX and isoproterenol (ISO). d, Net Gαi constitutive activity was calculated using formula: cAMP level (RLU) from control + ISO/cAMP level (RLU) from PTX + ISO × 100. The data in a and b represent the mean ± s.e.m. of at least four different experiments each in quadruplicate. The data in c and d represent the mean ± s.e.m. of 32 wells (n = 32). †Weak partial agonist activity of naltrindole at Gαi signalling was described previously29,30. e, Effect of sodium site mutations on basal β-arrestin signalling. Wild-type and mutant δ-ORs were assayed for β-arrestin recruitment as described in Methods. HTLA cells were transfected with 15 μg of receptor DNA per 15-cm dish and basal activity measured 48 h later. Data are presented relative to wild-type receptor using the means ± s.e.m. of at least four different experiments each in quadruplicate. *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2 and Supplementary References. (PDF 232 kb)
Rights and permissions
About this article
Cite this article
Fenalti, G., Giguere, P., Katritch, V. et al. Molecular control of δ-opioid receptor signalling. Nature 506, 191–196 (2014). https://doi.org/10.1038/nature12944
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12944
This article is cited by
-
Structure-based design of bitopic ligands for the µ-opioid receptor
Nature (2023)
-
Reduction of stress responses in honey bees by synthetic ligands targeting an allatostatin receptor
Scientific Reports (2022)
-
Insights into divalent cation regulation and G13-coupling of orphan receptor GPR35
Cell Discovery (2022)
-
Identification of G protein-coupled receptor 55 (GPR55) as a target of curcumin
npj Science of Food (2022)
-
Structure determination of inactive-state GPCRs with a universal nanobody
Nature Structural & Molecular Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.