Abstract
The central nervous system (CNS) requires a tightly controlled environment free of toxins and pathogens to provide the proper chemical composition for neural function. This environment is maintained by the ‘blood–brain barrier’ (BBB), which is composed of blood vessels whose endothelial cells display specialized tight junctions and extremely low rates of transcellular vesicular transport (transcytosis)1,2,3. In concert with pericytes and astrocytes, this unique brain endothelial physiological barrier seals the CNS and controls substance influx and efflux4,5,6. Although BBB breakdown has recently been associated with initiation and perpetuation of various neurological disorders, an intact BBB is a major obstacle for drug delivery to the CNS7,8,9,10. A limited understanding of the molecular mechanisms that control BBB formation has hindered our ability to manipulate the BBB in disease and therapy. Here we identify mechanisms governing the establishment of a functional BBB. First, using a novel tracer-injection method for embryos, we demonstrate spatiotemporal developmental profiles of BBB functionality and find that the mouse BBB becomes functional at embryonic day 15.5 (E15.5). We then screen for BBB-specific genes expressed during BBB formation, and find that major facilitator super family domain containing 2a (Mfsd2a) is selectively expressed in BBB-containing blood vessels in the CNS. Genetic ablation of Mfsd2a results in a leaky BBB from embryonic stages through to adulthood, but the normal patterning of vascular networks is maintained. Electron microscopy examination reveals a dramatic increase in CNS-endothelial-cell vesicular transcytosis in Mfsd2a−/− mice, without obvious tight-junction defects. Finally we show that Mfsd2a endothelial expression is regulated by pericytes to facilitate BBB integrity. These findings identify Mfsd2a as a key regulator of BBB function that may act by suppressing transcytosis in CNS endothelial cells. Furthermore, our findings may aid in efforts to develop therapeutic approaches for CNS drug delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Referenced accessions
Gene Expression Omnibus
Data deposits
Microarray data have been deposited in NCBI’s Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE56777.
References
Saunders, N. R., Liddelow, S. A. & Dziegielewska, K. M. Barrier mechanisms in the developing brain. Front. Pharmacol. 3, 46 (2012)
Reese, T. S. & Karnovsky, M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217 (1967)
Siegenthaler, J. A., Sohet, F. & Daneman, R. ‘Sealing off the CNS': cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol. 23, 1057–1064 (2013)
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010)
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010)
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 321–323 (2010)
Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008)
Zhong, Z. et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature Neurosci. 11, 420–422 (2008)
Bell, R. D. & Zlokovic, B. V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 118, 103–113 (2009)
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012)
Saunders, N. R. et al. Transporters of the blood–brain and blood–CSF interfaces in development and in the adult. Mol. Aspects Med. 34, 742–752 (2013)
Stenman, J. M. et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 (2008)
Liebner, S. et al. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 183, 409–417 (2008)
Daneman, R. et al. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl Acad. Sci. USA 106, 641–646 (2009)
Tam, S. J. et al. Death receptors DR6 and TROY regulate brain vascular development. Dev. Cell 22, 403–417 (2012)
Cullen, M. et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood–brain barrier. Proc. Natl Acad. Sci. USA 108, 5759–5764 (2011)
Wang, Y. et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344 (2012)
Alvarez, J. I. et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011)
Mizee, M. R. et al. Retinoic acid induces blood–brain barrier development. J. Neurosci. 33, 1660–1671 (2013)
Stern, L., Rapoport, J. L. & Lokschina, E. S. Le fonctionnement de la barrière hémato-encéphalique chez les nouveau-nés. C. R. Soc. Biol 100, 231–223 (1929)
Tang, T. et al. A mouse knockout library for secreted and transmembrane proteins. Nature Biotechnol. 28, 749–755 (2010)
Esnault, C. placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl Acad. Sci. USA 105, 17532–17537 (2008)
Reiling, J. H. et al. A Haploid genetic screen identifies the major facilitator domain containing2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc. Natl Acad. Sci. USA 108, 11756–11765 (2011)
Toufaily, C. et al. MFSD2a, the Syncytin-2 receptor, is important for trophoblast fusion. Placenta 34, 85–88 (2013)
Berger, J. H. Charron, M.J. Silver, D.L. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 7, e50629 (2012)
Nag, S. In The Blood–Brain Barrier—Biology and Research Protocols. Part 2 (ed. Nag, S. ). 99–100 (2003)
Chen, F., Evans, A., Pham, J. & Plosky, B. (eds) Mol. Cell Special Review issue. 40, (2010)
Ek, C. J., Habgood, M. D., Dziegielewska, K. M. & Saunders, N. R. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphisdomestica). J. Comp. Neurol. 496, 13–26 (2006)
Risau, W., Hallmann, R. & Albrecht, U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev. Biol. 117, 537–545 (1986)
Bauer, H. et al. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res. Dev. Brain Res. 86, 317–325 (1995)
Daneman, R. et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5, 313741 (2010)
Acknowledgements
We thank M. Karnovsky, E. Raviola and T. Reese for advice and discussion; S. R. Datta, C. Weitz, M. Greenberg, Q. Ma, C. Harvey and members of the Gu laboratory for comments on the manuscript; D. Sabatini and J. Reeling for sharing unpublished data; C. Betsholtz and C. Olsson for providing Pdgfbret/ret mouse brain samples; T. Schwarz and A. Oztan for discussion and advice on cell trafficking; W.-J. Oh for help with graphic illustrations; the Flow Cytometry Facility in the department of Systems Biology at Harvard Medical School for cell sorting; the Microarray Core at Dana-Farber Cancer Institute for Affymetrix assay; HSPH Bioinformatics Core, Harvard School of Public Health, for assistance with microarray analysis and Gene Expression Omnibus (GEO) submission; the Enhanced Neuroimaging Core at Harvard NeuroDiscovery Center for helping with confocal imaging and image analysis; the HMS Electron Microscopy Core Facility, the Neurobiology Imaging Facility for consultation and instrument availability that supported this work (this facility is supported in part by the Neural Imaging Center as part of an NINDS P30 Core Center grant no. NS072030); R. Polakiewicz and J. Xie from CST for generating Mfsd2a antibodies. This work was supported by the Harold Perlman postdoctoral fellowships, the Goldenson postdoctoral fellowship, and the Lefler postdoctoral fellowship (A.B.-Z.); the DFG-German Research Foundation postdoctoral fellowship (E.K.); the Mahoney postdoctoral fellowship (B.L.); NIH training grant 5T32MH20017-15 (B.J.A.); and the Sloan research fellowship, Armenise junior faculty award, the Genise Goldenson fund, the Freudenberger award, and NIH grant R01NS064583 (C.G.).
Author information
Authors and Affiliations
Contributions
C.G. and A.B.-Z. conceived and designed the project. A.B.-Z, B.L., E.K, B.J.A. and H.Y. performed experiments. A.B.-Z. and B.L. analysed data and preformed image analysis and quantification. Y.M. analysed microarray data. C.G. and A.B.-Z. wrote the manuscript with significant input from B.L. and B.J.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Diagram illustrating two unique BBB properties of CNS endothelial cells.
Compared to the endothelial cells from the rest of the body, CNS endothelial cells that possess a BBB are characterized by highly specialized tight junctions sealing the space between adjacent cells (1), and an unusually low rate of transcytosis from the vessel lumen to the brain parenchyma (2).
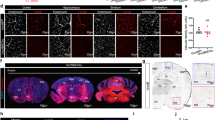
Extended Data Figure 2 Spatial and temporal BBB maturation across brain regions and cortical regions.
a, b, Embryonic BBB develops in a caudal-to-rostral spatial pattern. Dextran-tracer (10 kDa) injection revealed that the BBB is already functional in the hindbrain (a) and midbrain (b) at E14.5, a time-point at which cortical BBB is still leaky. Epifluorescence (low magnification) and confocal (high magnification) images of brain sections from injected embryos are shown. As illustrated in both a and b, tracer was confined to blood vessels (arrows). c–e, Cortical BBB develops in a ventrolateral to dorsomedial spatial pattern. c, Diagram of the embryonic cortex indicating dorsal–medial and ventral–lateral cortical regions illustrated in d and e. d, At E13.5, the BBB of the dorsal cortex (top panels) is not fully formed, as evidenced by little tracer inside the blood vessels (arrow) and tracer-filled parenchymal cells (arrowheads). In the ventral cortex (bottom panels), capillaries were better sealed, showing more tracer within the lumen (arrow) and less tracer in the brain parenchyma (arrowhead). e, At E14.5, the capillaries in the dorsal cortex (top panels) were still leaky, with little tracer inside the capillaries (arrow) and tracer-stained surrounding parenchyma (arrowheads). At the same age, the BBB of the ventral cortex (bottom panels) was already functional, with all tracer confined to the capillaries (arrow). Green, lectin; red, 10-kDa tracer. n = 6 embryos from 3 litters for each age.
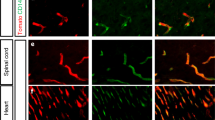
Extended Data Figure 3 Mfsd2a protein is selectively expressed in BBB-containing CNS vasculature of both embryos and adults.
Immunohistochemical staining of Mfsd2a protein demonstrating its specific expression in BBB-containing CNS vasculature. Red, Mfsd2a; green, PECAM (endothelium); blue, DAPI (nuclei). a, At E13.5, the time of BBB establishment, Mfsd2a expression was detected in the brain (top panels) but not in the lung vessels (bottom panels), confirming the microarray data. b, At E15.5,the first developmental time-point of BBB functionality, Mfsd2a expression was detected in spinal cord (top panels) but not in lung (middle panels) or liver vessels (bottom panels). c, Mfsd2a selective expression in BBB-containing vessels persists in adult mice (P90) as shown in brain vessels (top panels) but not in lung or liver (bottom panels). d, Mfsd2a is expressed in cerebral vessel (arrow) but not in pial vessels (arrow heads). Low (top panel) and high magnification (bottom panel) of pial-cerebral boundary (dotted line) of P5 dorsal cortex (wild-type mice). n = 3 embryos from 3 litters for each age.
Extended Data Figure 4 The leaky BBB phenotype in Mfsd2a−/− mice persists after birth and is not restricted to carbohydrate-based tracers.
a, b, Injection of two non-carbohydrate-based tracers with different molecular weight and different molecular compositions at P4 revealed a persistent leaky barrier phenotype in mice lacking Mfsd2a. a, The small-molecular-weight tracer sulfo-NHS-biotin (∼550 Da), was confined to vessels in wild-type controls (upper panels), whereas it leaked out of the vessels (arrowheads) in Mfsd2a−/− mice (lower panels). Green, lectin; red, tracer. b, The high-molecular-weight protein tracer HRP (∼44 kDa) was confined to vessels in control mice at P4 (left), whereas in mice lacking Mfsd2a (right), tracer was diffusing into the brain parenchyma (arrowheads). Brown, visualization of HRP in light microscopy by DAB reaction. Stainings were carried out on 100-µm cortical sections of tracer-injected animals. n = 4 pups per genotype from 3 litters. c, d, BBB leakage of tracer with 70 kDa molecular weight is observed in postnatal Mfsd2a−/− mice. Green, lectin or PECAM (vessels); red, tracer. The 10-kDa (a) and 70-kDa (b) tracers fluoro-ruby-dextran were confined to vessels in control mice (top panels), but not in Mfsd2a−/− mice (bottom panels), where tracer was taken up by brain parenchymal cells (arrowheads). Stainings were carried out on 12-µm cortical sections of tracer-injected animals. n = 3 pups per genotype from 3 litters.
Extended Data Figure 5 Perinatal and adult mice lacking Mfsd2a do not display changes in cerebrovascular network properties or signs of vascular degeneration.
a, No abnormalities were found in cortical capillary density and vascular branching (top panels) as well as capillary diameter (bottom panels) of postnatal (P4, left) and adult (P70, right) Mfsd2a−/− mice. Images of vascular staining in coronal cortical sections and high-magnification images of cross-section profiles of capillaries (green, PECAM) and quantification. Data are mean ± s.e.m. n = 3 animals per genotype, 20 sections per animal. b, Immunostaining for smooth-muscle actin (arterial identifier, arrows) revealed no abnormalities in arterial distribution and specification in Mfsd2a−/− mice. Images of coronal cortical sections (left, green, PECAM; red, SMA) and quantification (right). Data are mean ± s.e.m. n = 3 animals per genotype, 20 sections per animal. c, Electron-microscopy examination of older Mfsd2a−/− mice did not reveal signs of cerebrovascular degeneration. Left, the overall capillary structure (for example, cell size, shape of the nucleus, thickness of the vessel wall, basement membrane integrity and pericyte attachment) did not differ between wild-type and mutant mice. Right, at higher magnification, normal features, such as pericyte (asterisk) attachment within a normal basement membrane (between arrows), could be observed in mice lacking Mfsd2a.
Extended Data Figure 6 Pericyte coverage, attachment and ultrastructure are normal in Mfsd2a−/− mice.
a, b, Mfsd2a−/− mice exhibit normal pericyte coverage. Co-staining of endothelium (claudin-5, red) and pericytes (CD13 in a and Pdgfrβ in b, green) revealed no overt difference in pericyte coverage of dorsal cortex vessels between wild-type (top row) and Mfsd2a−/− (bottom row) mice at P5. Quantification of vascular coverage in both a and b showed no significant difference between wild-type and Mfsd2a−/− samples (P > 0.5). Data are mean ± s.e.m. n = 3 pups per genotype, 20 sections per animal. c, E17.5 Mfsd2a−/− mice exhibit normal pericyte–endothelial attachment. High magnification images of capillary cross sections co-staining for endothelium (Glut1, red) and pericytes (Pdgfrβ, green) revealed no difference in pericyte-endothelial relationships between wild-type (top panels) and Mfsd2a−/− (bottom panels) mice (endothelial nucleus and pericyte nucleus are indicated by single and double asterisks, respectively). d, Electron micrographs of longitudinal capillary sections revealed that pericytes had normal appearance and were well positioned on the vessel walls in Mfsd2a−/− adult mice; pericytes were adjacent to endothelial cells and shared a common basement membrane. L, lumen; P, pericyte.
Extended Data Figure 7 Mfsd2a gene expression and Mfsd2a protein levels are downregulated in mouse models of reduced pericyte coverage.
a, Analysis of microarray data5 showed high levels of Mfsd2a expression in wild-type adult brain microvasculature, but a significant decrease in levels of Mfsd2a expression in mice that have reduced pericyte coverage at the BBB. Pdgfbret/ret mice (mouse model 1), where Pdgfβ binding to heparan sulphate proteoglycans was disrupted, exhibited a major loss of pericyte coverage (74% reduction)5 and showed a dramatic decrease in Mfsd2a expression (74% reduction) compared to that of littermate controls. Similarly, Tie2Cre/R26P+/0, Pdgfb−/− mice (mouse model 2) in which Pdgfb-null alleles were complemented by one copy of human PDGFB transgene showed a less dramatic loss of pericyte coverage (60% reduction)5 and a smaller decrease in Mfsd2a expression (53% reduction). **P = 0.004, ***P = 1 × 10−5). Bars reflect normalized signal of the Mfsd2a probe (1428223_at) in adult brain or cortex microvascular fragments (a.u.). Data are mean ± s.d. of 4 biological replicates. b, Mfsd2a immunostaining in cerebrovasculature of postnatal Pdgfbret/ret mice and littermate controls (Pdgfbret/+) revealed reduced Mfsd2a protein expression in endothelial cells that are not covered by pericytes. Cross section: red, Mfsd2a; green, claudin-5 (endothelium); blue, DAPI (nuclei); grey, Pdgfrβ (pericyte). Reduced Mfsd2a signal was observed in endothelial cells of capillaries not covered with pericytes in Pdgfbret/ret mice (arrow, bottom panels), whereas strong Mfsd2a signal was apparent in pericyte-covered capillaries in Pdgfbret/+ mice (arrow, top panel). Insets in b′ demonstrate in high magnification that Mfsd2a expression is restricted to endothelial cells (co-localization with claudin-5 staining) and absent in pericytes (none co-localization with Pdgfrβ staining). c, Additional example of the reduction in Mfsd2a expression in endothelial cells of Pdgfbret/ret mice (longitudinal section). d, Quantification of mean fluorescence intensity per vascular profile showed significant reduction of Mfsd2a signal in Pdgfbret/ret capillaries compared to controls. Data are mean ± s.e.m. n = 2 animals per genotype, 60 images quantified of at least 600 vascular profiles per animal.
Extended Data Figure 8 Immuno-electron-microscopy reveals the subcellular localization of Mfsd2a on the plasma membrane and vesicles, but not in tight junctions of cerebral endothelial cells.
a, Electron micrographs showing silver-enhanced immunogold labelling of Mfsd2a in cerebral cortex capillaries from wild-type (left) but not in Mfsd2a−/− mice (right), demonstrating staining specificity. b, Top panels (i–iii), three representative examples of Mfsd2a localization on the plasma membrane (arrows) and in the cytoplasm (arrowheads), but not in tight junctions (asterisk). Bottom panels (iv–vi), high magnification representative examples of Mfsd2a localization on the luminal plasma membrane (arrows), associated with luminal invaginating vesicles (iv, v) and with cytoplasmic vesicles (arrowheads). All samples are of cortical vessels from adult mice (P30–P90). n = 2 for each genotype. L, lumen.
Supplementary information
Supplementary Information
This file contains Supplementary Data, a Supplementary Discussion and additional references. (PDF 168 kb)
Rights and permissions
About this article
Cite this article
Ben-Zvi, A., Lacoste, B., Kur, E. et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509, 507–511 (2014). https://doi.org/10.1038/nature13324
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13324
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
Endothelial and mural laminin-α5 contributes to neurovascular integrity maintenance
Fluids and Barriers of the CNS (2024)
-
Sex, hormones and cerebrovascular function: from development to disorder
Fluids and Barriers of the CNS (2024)
-
Entry and exit of extracellular vesicles to and from the blood circulation
Nature Nanotechnology (2024)
-
Identification of direct connections between the dura and the brain
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.