Abstract
The Ras-like GTPases RalA and RalB are important drivers of tumour growth and metastasis1. Chemicals that block Ral function would be valuable as research tools and for cancer therapeutics. Here we used protein structure analysis and virtual screening to identify drug-like molecules that bind to a site on the GDP-bound form of Ral. The compounds RBC6, RBC8 and RBC10 inhibited the binding of Ral to its effector RALBP1, as well as inhibiting Ral-mediated cell spreading of murine embryonic fibroblasts and anchorage-independent growth of human cancer cell lines. The binding of the RBC8 derivative BQU57 to RalB was confirmed by isothermal titration calorimetry, surface plasmon resonance and 1H–15N transverse relaxation-optimized spectroscopy (TROSY) NMR spectroscopy. RBC8 and BQU57 show selectivity for Ral relative to the GTPases Ras and RhoA and inhibit tumour xenograft growth to a similar extent to the depletion of Ral using RNA interference. Our results show the utility of structure-based discovery for the development of therapeutics for Ral-dependent cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 November 2014
Changes were made to the author list, affiliations, author contributions and the K value in Fig. 2f.
References
Lim, K. H. et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 16, 2385–2394 (2006)
Schubbert, S., Shannon, K. & Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nature Rev. Cancer 7, 295–308 (2007)
Tsimberidou, A. M., Chandhasin, C. & Kurzrock, R. Farnesyltransferase inhibitors: where are we now? Expert Opin. Investig. Drugs 19, 1569–1580 (2010)
Roberts, P. J. & Der, C. J. Targeting the Raf–MEK–ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 (2007)
Yap, T. A. et al. Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 8, 393–412 (2008)
Neel, N. F. et al. The RalGEF–Ral effector signaling network: the road less traveled for anti-Ras drug discovery. Genes Cancer 2, 275–287 (2011)
Awasthi, S., Sharma, R., Singhal, S. S., Zimniak, P. & Awasthi, Y. C. RLIP76, a novel transporter catalyzing ATP-dependent efflux of xenobiotics. Drug Metab. Dispos. 30, 1300–1310 (2002)
Oxford, G. et al. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res. 65, 7111–7120 (2005)
Lim, K. H. et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7, 533–545 (2005)
Camonis, J. H. & White, M. A. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 15, 327–332 (2005)
Zipfel, P. A. et al. Ral activation promotes melanomagenesis. Oncogene 29, 4859–4864 (2010)
Peschard, P. et al. Genetic deletion of RALA and RALB small GTPases reveals redundant functions in development and tumorigenesis. Curr. Biol. 22, 2063–2068 (2012)
Martin, T. D. & Der, C. J. Differential involvement of RalA and RalB in colorectal cancer. Small GTPases 3, 126–130 (2012)
Yin, J. et al. Activation of the RalGEF/Ral Pathway promotes prostate cancer metastasis to bone. Mol. Cell. Biol. 27, 7538–7550 (2007)
Smith, S. C. et al. Expression of Ral GTPases, their effectors, and activators in human bladder cancer. Clin. Cancer Res. 13, 3803–3813 (2007)
Smith, S. C., Baras, A. S., Owens, C. R., Dancik, G. & Theodorescu, D. Transcriptional signatures of Ral GTPase are associated with aggressive clinicopathologic characteristics in human cancer. Cancer Res. 72, 3480–3491 (2012)
Pautsch, A., Vogelsgesang, M., Trankle, J., Herrmann, C. & Aktories, K. Crystal structure of the C3bot–RalA complex reveals a novel type of action of a bacterial exoenzyme. EMBO J. 24, 3670–3680 (2005)
Shoichet, B. K. Virtual screening of chemical libraries. Nature 432, 862–865 (2004)
Irwin, J. J. & Shoichet, B. K. ZINC—a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 45, 177–182 (2005)
Balasubramanian, N. et al. RalA–exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr. Biol. 20, 75–79 (2010)
Fenwick, R. B. et al. Solution structure and dynamics of the small GTPase RalB in its active conformation: significance for effector protein binding. Biochemistry 48, 2192–2206 (2009)
Hinoi, T. et al. Post-translational modifications of Ras and Ral are important for the action of Ral GDP dissociation stimulator. J. Biol. Chem. 271, 19710–19716 (1996)
Fang, Z., Grutter, C. & Rauh, D. Strategies for the selective regulation of kinases with allosteric modulators: exploiting exclusive structural features. ACS Chem. Biol. 8, 58–70 (2013)
Sun, Q. et al. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew. Chem. Int. Edn Engl. 51, 6140–6143 (2012)
Maurer, T. et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc. Natl Acad. Sci. USA 109, 5299–5304 (2012)
Shima, F. et al. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc. Natl Acad. Sci. USA 110, 8182–8187 (2013)
Holbourn, K. P., Sutton, J. M., Evans, H. R., Shone, C. C. & Acharya, K. R. Molecular recognition of an ADP-ribosylating Clostridium botulinum C3 exoenzyme by RalA GTPase. Proc. Natl Acad. Sci. USA 102, 5357–5362 (2005)
Jin, R. et al. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 24, 2064–2074 (2005)
Fukai, S., Matern, H. T., Jagath, J. R., Scheller, R. H. & Brunger, A. T. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. EMBO J. 22, 3267–3278 (2003)
Thompson, G., Owen, D., Chalk, P. A. & Lowe, P. N. Delineation of the Cdc42/Rac-binding domain of p21-activated kinase. Biochemistry 37, 7885–7891 (1998)
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995)
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005)
Bahrami, A., Assadi, A. H., Markley, J. L. & Eghbalnia, H. R. Probabilistic interaction network of evidence algorithm and its application to complete labeling of peak lists from protein NMR spectroscopy. PLOS Comput. Biol. 5, e1000307 (2009)
Steggerda, S. M. & Paschal, B. M. The mammalian Mog1 protein is a guanine nucleotide release factor for Ran. J. Biol. Chem. 275, 23175–23180 (2000)
Acknowledgements
This work was supported in part by NIH grants CA091846, CA075115, CA104106 and GM47214 by the IUPUI Research Scholar Grant Foundation and by an American Cancer Society Research Scholar grant. The researchers used the services of the Medicinal Chemistry Core (MCC) facility (M.F.W.) housed within the Department of Pharmaceutical Sciences, University of Colorado. In part, the MCC is funded by Colorado Clinical and Translational Sciences Institute grant UL1TR001082 from the National Center for Research Resources, NIH. We acknowledge D. S. Backos for assistance with computational modelling, A. Spencer for biochemical assays, B. Helfrich for assistance with lung cancer cell line culturing, and H. Mo and J. Harwood for assistance in the training and collection of NMR data in the early stages of the project.
Author information
Authors and Affiliations
Contributions
D.T. and S.O.M. conceived of the initial screening concept. D.T. assembled the team and coordinated the project. C.Y., L.L., M.K., W.E.K., D.L., J.M., B.H., M.D.N., B.M.P., D.L.B., S.G., C.O. and C.L.W. performed experimental work and data analysis. M.F.W. performed and analysed the pharmacokinetic and pharmacodynamic experiments. D.N.M.J. performed and analysed the NMR experiments. M.A. performed GTP assays. D.T., C.Y., S.O.M., D.N.M.J., D.L.B., B.M.P. D.R. and M.A.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Structure model of RalB–GNP.
a, Ribbon model showing the switch-I and switch-II regions and the α2 and α3 helices. b, Surface model showing absence of the allosteric binding site. All models were generated with Accelrys Discovery Studio software using the published RalB–GNP structure (PDB ID, 2KE5).
Extended Data Figure 2 Cell-based secondary screening identified RBC6, RBC8 and RBC10 as lead compounds for Ral inhibition.
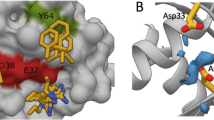
a, Scheme for the RalA activity ELISA assay. b, Examples of the effects of RBC6, RBC8 and RBC10 on the RalA-dependent spreading of MEFs. Wild-type or Cav−/− MEFs were treated with 15 µM compound for 1 h and then subjected to the MEF spreading assay, as described in Methods. c–e, Molecular docking of RBC6 (c), RBC8 (d) and RBC10 (e) into the target site of RalA–GDP. Compounds are shown in stick form and coloured purple (RBC6), cyan (RBC8) and pink (RBC10).
Extended Data Figure 3 NMR characterization of compound binding to Ral.
a, Plot of chemical shift differences between RalB–GDP and the previously published RalB–GNP structure (PDB ID, 2KE5) as a function of residue number. b, Mapping of chemical shift changes onto a homology model of the RalB–GDP complex (the model is based on RalA–GDP; PDB ID, 1U90). The mapping reveals that changes (magenta) mostly result from changes in the two loops that would otherwise bind to the third phosphate of GTP. GDP is shown as a stick model in cyan. c, 15N-TROSY spectrum of RalB–GDP (100 μM) in the absence (red) and presence (blue) of RBC8 (100 μM). Selected residues exhibiting significant chemical shift changes are also shown. d, Chemical shift changes in the RalB–GNP spectrum in the presence of RBC8 (100 μM). e, Chemical shift changes in the RalB–GDP (100 μM) spectrum in the presence of RBC5 (100 μM).
Extended Data Figure 4 Characterization of BQU57 binding to Ral.
a, Scheme for the chemical synthesis of BQU57. b, Chemical shift changes in RalB–GNP (100 μM) in the presence of BQU57 (100 μM). c, Plot of the 1H–15N-TROSY NMR chemical shift changes of selected residues in RalB–GDP with increasing concentrations of BQU57. d, Determination of the Kd for the binding of BQU57 to RalB–GDP using SPR. Top, SPR spectrum with increasing concentrations of BQU57; bottom, fitted binding curve, yielding a Kd of 4.7 μΜ.
Extended Data Figure 5 Activity of Ral inhibitors on human cancer cell lines in vitro.
a–c, Cellular uptake of Ral inhibitors in vitro. H2122 human lung cancer cells were treated with RBC5, RBC8 or BQU57 (10 μM). The cells were collected at various time points (1, 5, 15, 30 and 60 min), and the drug concentrations in cells were determined using LC-MS/MS methods. The data are presented as the mean ± s.d. of triplicate samples. d, Effect of RBC5 treatment on the anchorage-independent growth of H2122 and H358 human lung cancer cell lines. The cells were seeded in soft agar containing various concentrations of each drug; the colonies that formed in soft agar were counted after 2–4 weeks. The data are presented as the mean ± s.d. of triplicate samples. e, Inhibition of Ral activity in H2122 and H358 cells by RBC5, RBC8 and BQU57. The cells were grown under anchorage-independent conditions and treated with 10 μΜ compound for 3 h. The Ral activity in cell lysates was then determined using a pull-down assay with RALBP1–agarose beads. Total lysates (20 μg protein) and RALBP1 pull-downs (from 400 μg protein) were analysed by immunoblotting using antibodies specific for RalA and RalB. The data represent three independent experiments.
Extended Data Figure 6 RAS and RAL knockdown in human cancer cell lines.
a, b, Effect of K-RAS knockdown on anchorage-independent growth of four human lung cancer cell lines. Immunoblot showing siRNA-mediated knockdown of K-RAS in H2122, H358, H460 and Calu-6 cell lines 48 h after siRNA transfection (a). All four cell lines were sensitive to K-RAS knockdown, as determined using the soft agar colony formation assay (b). The data are presented as the mean ± s.d. of triplicate samples. *, P < 0.05, Student’s t-test. c, d, Effect of RAL knockdown on anchorage-independent growth of four human lung cancer cell lines. The cells were transfected with siRNA directed against RALA, RALB or RALA and RALB (RalA/B) for 48 h and then subjected to the soft agar colony formation assay. H2122 and H358 (c) but not H460 or Calu-6 (d) were sensitive to RAL knockdown. The data are presented as the mean ± s.d. of triplicate samples. *, P < 0.05, Dunnett’s test. e, Immunoblots showing knockdown of both RALA and RALB in H2122 and H358 cell lines 48 h after treatment with various concentrations of siRNA. f, Immunoblots showing successful overexpression of constitutively active RalAG23V or RalBG23V in H2122 and H358 cells. H2122 cells were transiently transfected with Flag, Flag–RalAG23V or Flag–RalBG23V for 48 h. H358 cells stably overexpressing Flag, Flag–RalAG23V and Flag–RalBG23V were generated by selection on the antibiotic G418.
Extended Data Figure 7 Effect of Ral inhibitors on xenograft models of human lung cancer.
a, Summary of the pharmacokinetic parameters of RBC8 and BQU57 in nude mice. Pharmacokinetic parameters were measured based on plasma levels after administration of a single intraperitoneal dose of 50 mg Ral inhibitor per kg body weight. AUC0–5 h, area under the curve, 0 to 5 h; Co, extrapolated initial concentration; T1/2, half-life. b, c, Tissue distribution of RBC8 (b) and BQU57 (c) in nude mice 3 h after a single intraperitoneal dose of 50 mg Ral inhibitor per kg body weight. The data are presented as the mean ± s.d. for three mice. d, RBC8 (50 mg per kg body weight per day), initiated 24 h after inoculation, inhibited the growth of tumour xenografts of the human lung cancer cell line H2122. Typical tumour appearance at 21 days is shown. e, Effect of RBC8 on H358 xenograft models. RBC8 treatment (50 mg per kg body weight per day), initiated 24 h after inoculation, inhibited the growth of tumour xenografts of the human lung cancer cell line H358. The data are presented as the mean ± s.e.m. for six mice. The tumour volume in the treatment group was significantly different from that in the control group, as determined by Student’s t-test (*, P < 0.05).
Extended Data Figure 8 Inhibition of Ral activity by RBC8 and RBC5 in vivo.
a–d, RBC8 inhibited RalA (a, b) and RalB (c, d) activity in H2122 tumour xenografts. Tumour-bearing nude mice were given a single dose of 50 mg RBC8 per kg body weight. After 3 h, the tumours were collected, and the Ral activity in tumour lysates was measured using the RALBP1 pull-down assay. Immunoblots from the Ral activity pull-down assay (a, c) and their quantification (b, d) are shown. Each lane represents one tumour sample. Each blot represents one treatment. The last lane in each blot (labelled LC, loading control) was loaded with 10 ng recombinant human RalA or RalB as an internal control for normalization and cross-blot comparison. The band intensity on each blot was first normalized to the internal control and then compared across different blots. The Ral activities in the treatment groups were significantly different from those in the controls, as determined by Student’s t-test (*, P < 0.001, n = 24). e, f, RBC5 did not inhibit RalA (e) or RalB (f) activity in H2122 tumour xenografts. Tumour-bearing nude mice were given a single dose of 50 mg RBC5 per kg body weight. After 3 h, the tumours were collected, and the Ral activity in tumour lysates was measured using the RALBP1 pull-down assay (n = 6).
Supplementary information
Supplementary Information
This file contains Supplementary Methods describing the chemical synthesis pathway for BQU57 and Supplementary Table 1, which shows that none of the 88 compounds identified by computational screening inhibited GTP or GDP binding to purified recombinant RalA. (PDF 146 kb)
Rights and permissions
About this article
Cite this article
Yan, C., Liu, D., Li, L. et al. Discovery and characterization of small molecules that target the GTPase Ral. Nature 515, 443–447 (2014). https://doi.org/10.1038/nature13713
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13713
This article is cited by
-
A RalA between high-fat diet and mitochondrial shape
Nature Metabolism (2024)
-
Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation
Nature Metabolism (2024)
-
Regulation of Ras p21 and RalA GTPases activity by quinine in mammary epithelial cells
Molecular and Cellular Biochemistry (2023)
-
The role of ral signaling and post translational modifications (PTMs) of Ras in cancer
Genome Instability & Disease (2022)
-
Lnc00892 competes with c-Jun to block NCL transcription, reducing the stability of RhoA/RhoC mRNA and impairing bladder cancer invasion
Oncogene (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.