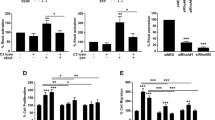
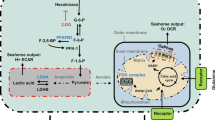
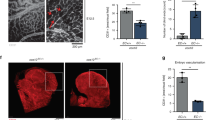
Abstract
The metabolism of endothelial cells during vessel sprouting remains poorly studied. Here we report that endothelial loss of CPT1A, a rate-limiting enzyme of fatty acid oxidation (FAO), causes vascular sprouting defects due to impaired proliferation, not migration, of human and murine endothelial cells. Reduction of FAO in endothelial cells did not cause energy depletion or disturb redox homeostasis, but impaired de novo nucleotide synthesis for DNA replication. Isotope labelling studies in control endothelial cells showed that fatty acid carbons substantially replenished the Krebs cycle, and were incorporated into aspartate (a nucleotide precursor), uridine monophosphate (a precursor of pyrimidine nucleoside triphosphates) and DNA. CPT1A silencing reduced these processes and depleted endothelial cell stores of aspartate and deoxyribonucleoside triphosphates. Acetate (metabolized to acetyl-CoA, thereby substituting for the depleted FAO-derived acetyl-CoA) or a nucleoside mix rescued the phenotype of CPT1A-silenced endothelial cells. Finally, CPT1 blockade inhibited pathological ocular angiogenesis in mice, suggesting a novel strategy for blocking angiogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
05 August 2015
A Correction to this paper has been published: https://doi.org/10.1038/nature14624
References
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011)
Ausprunk, D. H. & Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 14, 53–65 (1977)
Welti, J., Loges, S., Dimmeler, S. & Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 123, 3190–3200 (2013)
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013)
De Bock, K., Georgiadou, M. & Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 18, 634–647 (2013)
Colavitti, R. et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 277, 3101–3108 (2002)
Fendt, S. M. et al. Reductive glutamine metabolism is a function of the α-ketoglutarate to citrate ratio in cells. Nat. Commun. 4, 2236 (2013)
Vander Heiden, M. G. Exploiting tumor metabolism: challenges for clinical translation. J. Clin. Invest. 123, 3648–3651 (2013)
Thompson, C. B. Wnt meets Warburg: another piece in the puzzle? EMBO J. 33, 1420–1422 (2014)
Carracedo, A., Cantley, L. C. & Pandolfi, P. P. Cancer metabolism: fatty acid oxidation in the limelight. Nature Rev. Cancer 13, 227–232 (2013)
Henry, T. D., Satran, D. & Jolicoeur, E. M. Treatment of refractory angina in patients not suitable for revascularization. Nature Rev. Cardiol. 11, 78–95 (2014)
Morris, G. W., Iams, T. A., Slepchenko, K. G. & McKee, E. E. Origin of pyrimidine deoxyribonucleotide pools in perfused rat heart: implications for 3′-azido-3′-deoxythymidine-dependent cardiotoxicity. Biochem. J. 422, 513–520 (2009)
Fairbanks, L. D., Bofill, M., Ruckemann, K. & Simmonds, H. A. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J. Biol. Chem. 270, 29682–29689 (1995)
Lunt, S. Y. et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol. Cell 57, 95–107 (2015)
Cheung, E. C. et al. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev. Cell 25, 463–477 (2013)
Jaffe, E. A., Nachman, R. L., Becker, C. G. & Minick, C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 (1973)
Michieli, P. et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 6, 61–73 (2004)
Carlotti, F. et al. Lentiviral vectors efficiently transduce quiescent mature 3T3-L1 adipocytes. Mol. Ther. 9, 209–217 (2004)
Geudens, I. et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler. Thromb. Vasc. Biol. 30, 1695–1702 (2010)
Korff, T., Krauss, T. & Augustin, H. G. Three-dimensional spheroidal culture of cytotrophoblast cells mimics the phenotype and differentiation of cytotrophoblasts from normal and preeclamptic pregnancies. Exp. Cell Res. 297, 415–423 (2004)
Schoors, S. et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 19, 37–48 (2014)
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nature Med. 7, 575–583 (2001)
Dagher, Z., Ruderman, N., Tornheim, K. & Ido, Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 88, 1276–1282 (2001)
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011)
Aragonés, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nature Genet. 40, 170–180 (2008)
Fendt, S. M. et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 73, 4429–4438 (2013)
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Elementary metabolite units (EMU): a novel framework for modeling isotopic distributions. Metab. Eng. 9, 68–86 (2007)
Fernandez, C. A., Des Rosiers, C., Previs, S. F., David, F. & Brunengraber, H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J. Mass Spectrom. 31, 255–262 (1996)
Nanchen, A., Fuhrer, T. & Sauer, U. Determination of metabolic flux ratios from 13C-experiments and gas chromatography-mass spectrometry data: protocol and principles. Methods Mol. Biol. 358, 177–197 (2007)
Wilson, P. M. et al. A novel fluorescence-based assay for the rapid detection and quantification of cellular deoxyribonucleoside triphosphates. Nucleic Acids Res. 39, e112 (2011)
Sakaue-Sawano, A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008)
Millard, P., Letisse, F., Sokol, S. & Portais, J. C. IsoCor: correcting MS data in isotope labeling experiments. Bioinformatics 28, 1294–1296 (2012)
Büscher, J. M., Czernik, D., Ewald, J. C., Sauer, U. & Zamboni, N. Cross-platform comparison of methods for quantitative metabolomics of primary metabolism. Anal. Chem. 81, 2135–2143 (2009)
Benedito, R. et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 (2009)
Scott, A. & Fruttiger, M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye 24, 416–421 (2010)
Acknowledgements
We thank M. Vander Heiden and D. Tollervey for discussion, R. Adams for providing VE-cadherin(PAC)-CreERT2 mice, and S. Rodríguez-Arístegui for synthesis of etomoxir. S.S. is funded by the Institution of Research/Innovation (IWT); R.M., B.G., A.R.C. and J.G. by the Research Foundation Flanders (FWO); U.B. by a Marie Curie-IEF Fellowship; K.C.S.Q. by CAPES (Brazil) and G.B. by KU Leuven. The work of S.-M.F. is supported by Marie Curie CIG, FWO-OdysseusII, Concern Foundation, Bayer Healthcare Pharmaceuticals. The work of P.C. is supported by IUAP7/03, Methusalem funding (Flemish Government), FWO grants, Foundation Leducq Transatlantic Network (ARTEMIS), Foundation against Cancer, European Research Council (ERC) Advanced Research Grant (EU-ERC269073) and AXA Research grant. S.Y.L. was supported by the Department of Defense CDMRP Visionary Postdoctoral Award (W81XWH-12-1-0466). Views and opinions of, and endorsements by, the authors do not reflect those of the US Army or the Department of Defense. The authors thank the MSU LC-MS Core.
Author information
Authors and Affiliations
Contributions
S.S., U.B., R.M., K.C.S.Q., G.B., I.E., A.Z., A.R.C., S.C., J.G., W.H., L.G., S.V., P.P.V.V., G.E., L.S., M.D., M.B., K.D.B., B.G., S.Y.L., S.-M.F. and P.C. contributed to the performance of the experiments and/or analysis of the data; H.G. provided advice; S.S., U.B., K.D.B., S.M.F. and P.C. designed the experiments; S.S., M.D., K.D.B., S.M.F. and P.C. wrote the paper; S.M.F. and P.C. conceptualized the metabolic analysis, and P.C. conceived and directed the study. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.C. declares to be named as inventor on patent applications, claiming subject matter related to the results described in this paper.
Extended data figures and tables
Extended Data Figure 1 FAO regulates vessel sprouting.
a, mRNA expression of CPT1 isoforms (n = 3). b, CPT1A mRNA levels upon CPT1A silencing (CPT1AKD) (n = 11). c, Representative immunoblot of CPT1A for control and CPT1AKD ECs. d, FAO flux upon CPT1A silencing in venous (HUV) and arterial (HA) ECs, or upon small interference RNA transfection in venous ECs (siRNA) (n = 6 for HUV shRNA, n = 3 for HA shRNA and HUV siRNA). e, Schematic representation of FAO measurement using [9,10-3H]palmitate (reprinted from Immunity 35, Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation, 871–882 (2011), with permission from Elsevier; ref. 24). f, Representative immunoblot for CPT1A upon genetic silencing of CPT1A using siRNA (siCPT1A). g, FAO flux upon silencing of CPT1C (CPT1CKD) (n = 3; P = NS). h, FAO flux levels in venous (HUV), arterial (HA) and microvascular (HMV) ECs (n = 4 for HUV versus HA and n = 3 for HUV versus HMV). i, Sprout number in control and CPT1AKD EC spheroids with mitomycin C (MitoC) treatment as indicated (n = 3). j, Flow cytometry counting of viable control and CPT1AKD ECs (n = 3). k, Analysis of random cell-motility tracks in control and CPT1AKD ECs (n = 4; P = NS). l, FAO flux and proliferation upon silencing of ACADVL (ACADVLKD) (n = 3 for each). m, Wound closure in control and ACADVLKD ECs (n = 3; P = NS). n, o, Quantification of vessel sprouting in control and ACADVLKD EC spheroids with MitoC treatment as indicated, total sprout length (n) and sprout numbers per spheroid (o) (n = 5). p, Sprout number in control and CPT1AOE EC spheroids with MitoC treatment as indicated (n = 3). q, Total sprout length in control and CPT1AOE EC spheroids treated with MitoC as indicated (n = 5). r, s, Representative phase (original magnification, 10× magnification) contrast images of control (r) and CPT1AOE (s) EC spheroids. t, Scratch wound assay in control and CPT1AOE ECs treated with MitoC as indicated (n = 3; P = NS). u, PCR analysis of genomic DNA from WT and CPT1AΔEC pups, confirming Cre-mediated recombination of the floxed Cpt1a allele as shown by the appearance of a 300-bp band. v, NG2+ area in neonatal vascular plexus of WT and CPT1AΔEC mice (3 litters, n = 8 pups for WT and 7 pups for CPT1AΔEC; P = NS). Data are mean ± s.e.m. of n independent experiments (a, b, d, g–q, t) or the total number of mice (v). Statistical test: mixed models. NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Figure 2 CPT1A silencing does not cause cellular distress.
a, ADP/ATP ratio in control and CPT1AKD ECs (n = 3; P = NS). b, Sprout number upon oligomycin treatment (oligo) in control and CPT1AKD EC spheroids (n = 3). c, Glycolysis measurement in control and CPT1AKD ECs (n = 3; P = NS). d, Representative immunoblot for phosphorylated AMPK (pAMPK) and total AMPK (AMPK) and for LC3b I and II in control and CPT1AKD ECs. The ratio of the densitometrically quantified bands of pAMPK/AMPK and LC3b II/I is shown below the blots (n = 3; P = NS). e, Sprout number upon N-acetyl-cysteine (NAC) treatment in control and CPT1AKD EC spheroids (n = 3). f, g, Representative images (original magnification, 10×) of EC spheroids upon staining for TO-PRO3 in control (f) and CPT1AKD spheroids (g). h, i, Representative pictures (original magnification, 10×) of Hoechst/cleaved-caspase-3-stained control (h) and CPT1AKD (i) ECs. j, Representative immunoblots showing the ratio of phosphorylated (pATM)/total-ATM (ATM), p21/lamin and p53/lamin in control and CPT1AKD ECs. The ratios of the densitometrically quantified bands are shown below the blots (n = 3; P = NS). Data are mean ± s.e.m. of n independent experiments (a–e, j). Statistical test: mixed models. NS, not significant. ****P < 0.0001.
Extended Data Figure 3 FAO is used for de novo nucleotide synthesis.
a, Schematic representation of the different carbon sources used for de novo synthesis of UMP. Note that palmitate contributes three carbons to the nine carbon skeleton of UMP. PRPP, 5-phosphoribosyl-1-pyrophosphate. b, Sprout number upon 5-fluorouracil (5FU) treatment in control and CPT1AKD EC spheroids (n = 4). c, Sprout number upon methotrexate (MTX) treatment in control and CPT1AKD EC spheroids (n = 4). d, Sprout number upon acetate treatment in control and CPT1AKD EC spheroids (n = 3). e–g, Representative images (original magnification, 10×) of EC spheroids upon acetate treatment. h, i, Rescue of the sprouting defect of CPT1AKD spheroids by acetate was not affected by oligomycin treatment; panel h, total sprout length; panel i, sprout numbers/spheroid (n = 3; P = NS). j, k, Quantification of vessel sprouting using the EC spheroid model, showing that the reduction of total sprout length (j) and number of sprouts per spheroid (k) upon CPT1A silencing (CPT1AKD) was rescued by supplementation with a dNTP mix (n = 3). l, Quantification of MitoC-treated EC spheroid sprouting upon acetate or nucleoside mix supplementation (n = 3; P = NS). m, Glucose oxidation in ECs, measured by 14CO2 formation from [6-14C]glucose in control and CPT1AKD ECs (n = 4). n, Glutamine oxidation in ECs, measured by 14CO2 formation from [U-14C]glutamine in control and CPT1AKD ECs (n = 4; P = NS). o, Total contribution of [U-13C]glucose and [U-13C]glutamine to aspartate in control and CPT1AKD ECs (n = 3). p, [8-14C]hypoxanthine incorporation in RNA and DNA in control ECs (n = 3). Data are mean ± s.e.m. of n independent experiments (b–d, h–p). Statistical test: mixed models. NS, not significant. *P < 0.05, **P < 0.01, ****P < 0.0001.
Extended Data Figure 4 Etomoxir reduces vessel sprouting.
a, FAO flux upon etomoxir (Eto) treatment (n = 6). b, [3H]thymidine incorporation upon etomoxir treatment (n = 5). c, Scratch wound assay using MitoC-treated ECs upon etomoxir (Eto) treatment (n = 4; P = NS). d, Branch point quantification in the retinal vasculature of control (Ctrl) and etomoxir-treated (Eto) pups (8 litters, n = 24 pups for control and 16 for etomoxir treatment). e, f, Representative confocal images (original magnification, 10×) of retinal vessels stained for isolectin-B4 in control (e) and etomoxir (f) treated pups. g, Filopodia quantification in the retinal vascular front of control and etomoxir (Eto) treated pups (4 litters, n = 11 for WT and 9 for etomoxir; P = NS). Data are mean ± s.e.m. of n independent experiments (a–d, g) or the total number of mice (d, g). Statistical test: mixed models. NS, not significant. ****P < 0.0001.
Extended Data Figure 5 Analysis of steady state.
Percentage M+2 or M+4 citrate and aspartate over different time points (24, 36, 48 and 52 h) after labelling with [U-13C]glucose (a), [U-13C]glutamine (b), or [U-13C]palmitate (c). Data are mean ± s.d. of n = 3 independent experiments.
Rights and permissions
About this article
Cite this article
Schoors, S., Bruning, U., Missiaen, R. et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520, 192–197 (2015). https://doi.org/10.1038/nature14362
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14362
This article is cited by
-
CPT1A as a potential therapeutic target for lipopolysaccharide-induced acute lung injury in mice
Scientific Reports (2024)
-
VNS improves VSMC metabolism and arteriogenesis in infarcted hearts through m/n-AChR-Akt-SDF-1α in adult male rats
Journal of Molecular Histology (2024)
-
Molecular signatures of angiogenesis inhibitors: a single-embryo untargeted metabolomics approach in zebrafish
Archives of Toxicology (2024)
-
The PI3K-Akt-mTOR pathway mediates renal pericyte-myofibroblast transition by enhancing glycolysis through HKII
Journal of Translational Medicine (2023)
-
Molecular and metabolic orchestration of the lymphatic vasculature in physiology and pathology
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.