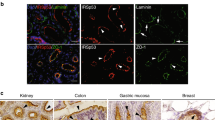
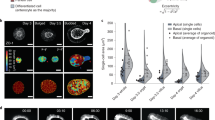
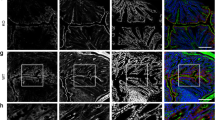
Abstract
The microvillus brush border at the apex of the highly polarized enterocyte allows the regulated uptake of nutrients from the intestinal lumen. Here, we identify the small G protein Rap2A as a molecular link that couples the formation of microvilli directly to the preceding cell polarization. Establishment of apicobasal polarity, which can be triggered by the kinase LKB1 in single, isolated colon cells, results in enrichment of PtdIns(4,5)P2 at the apical membrane. The subsequent recruitment of phospholipase D1 allows polarized accumulation of phosphatidic acid, which provides a local cue for successive signalling by the guanine nucleotide exchange factor PDZGEF, the small G protein Rap2A, its effector TNIK, the kinase MST4 and, ultimately, the actin-binding protein Ezrin. Thus, epithelial cell polarization is translated directly into the acquisition of brush borders through a small G protein signalling module whose action is positioned by a cortical lipid cue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
17 July 2012
In the version of this article initially published online, an arrowhead in Fig 6f was incorrectly placed. This has been corrected.
References
McCaffrey, L. M. & Macara, I. G. Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harb. Perspect. Biol. 1, a001370 (2009).
Zeqiraj, E., Filippi, B. M., Deak, M., Alessi, D. R. & van Aalten, D. M. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 326, 1707–1711 (2009).
Baas, A. F. et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 22, 3062–3072 (2003).
Boudeau, J. et al. MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114 (2003).
Dorfman, J. & Macara, I. G. STRADα regulates LKB1 localization by blocking access to importin-α, and by association with Crm1 and exportin-7. Mol. Biol. Cell 19, 1614–1626 (2008).
Baas, A. F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004).
Alessi, D. R., Sakamoto, K. & Bayascas, J. R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75, 137–163 (2006).
Park, H. O., Chant, J. & Herskowitz, I. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365, 269–274 (1993).
Pruyne, D., Legesse-Miller, A., Gao, L., Dong, Y. & Bretscher, A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20, 559–591 (2004).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Ten Klooster, J. P. et al. MST4 and Ezrin induce brush borders downstream of the LKB1/STRAD/MO25 polarization complex. Dev. Cell 16, 551–562 (2009).
Fehon, R. G., McClatchey, A. I. & Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 (2010).
Machida, N. et al. Mitogen-activated protein kinase kinase kinase kinase 4 as a putative effector of Rap2 to activate the c-Jun N-terminal kinase. J. Biol. Chem. 279, 15711–15714 (2004).
Taira, K. et al. The Traf2- and Nck-interacting kinase as a putative effector of Rap2 to regulate actin cytoskeleton. J. Biol. Chem. 279, 49488–49496 (2004).
Pannekoek, W. J. et al. Epac1 and PDZ-GEF cooperate in Rap1 mediated endothelial junction control. Cell Signal 23, 2056–2064 (2011).
Shewan, A., Eastburn, D. J. & Mostov, K. Phosphoinositides in cell architecture. Cold Spring Harb. Perspect. Biol. 3, a004796 (2011).
Van den Bout, I. & Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J. Cell Sci. 122, 3837–3850 (2009).
Sciorra, V. A. et al. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 18, 5911–5921 (1999).
Hodgkin, M. N. et al. Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-biphosphate-specific PH domain. Curr. Biol. 10, 43–46 (2000).
Du, G. et al. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J. Cell Biol. 162, 305–315 (2003).
Zeniou-Meyer, M. et al. Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J. Biol. Chem. 282, 21746–21757 (2007).
Nonaka, H. et al. MINK is a Rap2 effector for phosphorylation of thepostsynaptic scaffold protein TANC1. Biochem. Biophys. Res. Commun. 377, 573–578 (2008).
Gobel, V., Barrett, P. L., Hall, D. H. & Fleming, J. T. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6, 865–873 (2004).
Martin-Belmonte, F. et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 (2007).
Zhao, C., Du, G., Skowronek, K., Frohman, M. A. & Bar-Sagi, D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat. Cell Biol. 9, 706–712 (2007).
Gureasko, J. et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat. Struct. Mol. Biol. 15, 452–461 (2008).
Nishikimi, A. et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 324, 384–387 (2009).
Matsuki, T. et al. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell 143, 826–836 (2010).
Preisinger, C. et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J. Cell Biol. 164, 1009–1020 (2004).
Katagiri, K., Imamura, M. & Kinashi, T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 7, 919–928 (2006).
Schwamborn, J. C. & Puschel, A. W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 7, 923–929 (2004).
Lee, K. J. et al. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron 69, 957–973 (2011).
Dube, N. et al. The RapGEF PDZ-GEF2 is required for maturation of cell–cell junctions. Cell Signal 20, 1608–1615 (2008).
Ponsioen, B. et al. Direct spatial control of Epac1 by cAMP. Mol. Cell Biol. 29, 2521–2531 (2009).
Reedquist, K. A. et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148, 1151–1158 (2000).
Van der Wal, J., Habets, R., Varnai, P., Balla, T. & Jalink, K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276, 15337–15344 (2001).
Varnai, P. et al. Inositol lipid binding and membrane localization of isolated pleckstrin homology (PH) domains. Studies on the PH domains of phospholipase C delta 1 and p130. J. Biol. Chem. 277, 27412–27422 (2002).
Lewis, J. A. & Fleming, J. T. Basic culture methods. Methods Cell Biol. 48, 3–29 (1995).
Van Triest, M., de Rooij, J. & Bos, J. L. Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol. 333, 343–348 (2001).
Fang, Y., Vilella-Bach, M., Bachmann, R., Flanigan, A. & Chen, J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294, 1942–1945 (2001).
Van Galen, J. et al. Binding of GAPR-1 to negatively charged phospholipid membranes: unusual binding characteristics to phosphatidylinositol. Mol. Membr. Biol. 27, 81–91.
Acknowledgements
We are grateful to R. Wedlich-Söldner (Max Planck Institute of Biochemistry, Martinsried, Germany), N. Vitale (Institut des Neurosciences Cellulaires et Intégratives, Strasbourg, France), T. Balla (National Institutes of Health, Bethesda, USA), J. Chen (University of Illinois, Urbana, USA) and S. van den Heuvel (Universiteit Utrecht, Utrecht, The Netherlands) for providing reagents. We thank W. Vonk, L. Kleij and K. Lee for technical assistance, C. Garner for assistance with lentiviral infections, K. Jalink for discussions, G. Kops, T. Pawson and J. Nelson for critical reading of the manuscript, and the members of our laboratories for continuous support and stimulating discussions. This study is supported by the Dutch Cancer Society (KWF; J.P.t.K. and M.J.V.), Chemical Sciences (M.G.) and the Netherlands Genomics Initiative (J.L.B. and H.C.) of the Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Contributions
M.G., J.P.t.K. and J.L.B. designed the study and experiments; M.G., J.P.t.K., M.J.V., T.K. and F.J.Z. performed experiments; M.G. and J.L.B. wrote the manuscript; H.C. and J.L.B. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 789 kb)
Supplementary Movie 1
(MPG 678 kb)
Supplementary Movie 2
(MPG 678 kb)
Rights and permissions
About this article
Cite this article
Gloerich, M., ten Klooster, J., Vliem, M. et al. Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 14, 793–801 (2012). https://doi.org/10.1038/ncb2537
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2537
This article is cited by
-
Discrete localization of phospholipase Cβ3 and diacylglycerol kinase ι along the renal proximal tubules of normal rat kidney and gentamicin-induced changes in their expression
Histochemistry and Cell Biology (2023)
-
Molecular Alterations in Malignant Pleural Mesothelioma: A Hope for Effective Treatment by Targeting YAP
Targeted Oncology (2022)
-
RAP2 mediates mechanoresponses of the Hippo pathway
Nature (2018)
-
Differential regulation of spermatogenic process by Lkb1 isoforms in mouse testis
Cell Death & Disease (2017)
-
Dynamic relocalization of NHERF1 mediates chemotactic migration of ovarian cancer cells toward lysophosphatidic acid stimulation
Experimental & Molecular Medicine (2017)