Abstract
A major obstacle to understanding the functional importance of dimerization between class A G protein–coupled receptors (GPCRs) has been the methodological limitation in achieving control of the identity of the components comprising the signaling unit. We have developed a functional complementation assay that enables such control, and we demonstrate it here for the human dopamine D2 receptor. The minimal signaling unit, two receptors and a single G protein, is maximally activated by agonist binding to a single protomer, which suggests an asymmetrical activated dimer. Inverse agonist binding to the second protomer enhances signaling, whereas agonist binding to the second protomer blunts signaling. Ligand-independent constitutive activation of the second protomer also inhibits signaling. Thus, GPCR dimer function can be modulated by the activity state of the second protomer, which for a heterodimer may be altered in pathological states. Our new methodology also makes possible the characterization of signaling from a defined heterodimer unit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pin, J.P. et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 59, 5–13 (2007).
Sartania, N., Appelbe, S., Pediani, J.D. & Milligan, G. Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric beta2-adrenoceptor. Cell. Signal. 19, 1928–1938 (2007).
Parenty, G., Appelbe, S. & Milligan, G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem. J. 412, 245–256 (2008).
Vilardaga, J.P. et al. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat. Chem. Biol. 4, 126–131 (2008).
Pin, J.P., Galvez, T. & Prezeau, L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 98, 325–354 (2003).
Brock, C. et al. Activation of a dimeric metabotropic glutamate receptor by intersubunit rearrangement. J. Biol. Chem. 282, 33000–33008 (2007).
Ji, I.H., Lee, C., Song, Y.S., Conn, P.M. & Ji, T.H. Cis- and trans-activation of hormone receptors: the LH receptor. Mol. Endocrinol. 16, 1299–1308 (2002).
Bayburt, T.H., Leitz, A.J., Xie, G., Oprian, D.D. & Sligar, S.G. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 (2007).
Whorton, M.R. et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 104, 7682–7687 (2007).
George, S.R. et al. Oligomerization of mu- and delta-opioid receptors-generation of novel functional properties. J. Biol. Chem. 275, 26128–26135 (2000).
Lee, S.P. et al. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678 (2004).
Guo, W., Shi, L. & Javitch, J.A. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 278, 4385–4388 (2003).
Mesnier, D. & Baneres, J.L. Cooperative conformational changes in a G-protein-coupled receptor dimer, the leukotriene B4 receptor BLT1. J. Biol. Chem. 279, 49664–49670 (2004).
Damian, M., Mary, S., Martin, A., Pin, J.P. & Baneres, J.L. G protein activation by the leukotriene B4 receptor dimer: evidence for an absence of trans-activation. J. Biol. Chem. 283, 21084–21092 (2008).
Carrillo, J.J., Pediani, J. & Milligan, G. Dimers of class A G protein-coupled receptors function via agonist-mediated trans-activation of associated G proteins. J. Biol. Chem. 278, 42578–42587 (2003).
Molinari, P. et al. Promiscuous coupling at receptor-Galpha fusion proteins. The receptor of one covalent complex interacts with the alpha -subunit of another. J. Biol. Chem. 278, 15778–15788 (2003).
Pascal, G. & Milligan, G. Functional complementation and the analysis of opioid receptor homodimerization. Mol. Pharmacol. 68, 905–915 (2005).
Seifert, R., Wenzel-Seifert, K. & Kobilka, B.K. GPCR-G[alpha] fusion proteins: molecular analysis of receptor-G-protein coupling. Trends Pharmacol. Sci. 20, 383–389 (1999).
Lee, T.W., Seifert, R., Guan, X. & Kobilka, B.K. Restricting the mobility of Gs alpha: impact on receptor and effector coupling. Biochemistry 38, 13801–13809 (1999).
Wenzel-Seifert, K., Lee, T.W., Seifert, R. & Kobilka, B.K. Restricting mobility of Gsalpha relative to the beta2-adrenoceptor enhances adenylate cyclase activity by reducing Gsalpha GTPase activity. Biochem. J. 334, 519–524 (1998).
Rizzuto, R., Simpson, A.W.M., Brini, M. & Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–327 (1992).
Blanpain, C. et al. Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J. Biol. Chem. 274, 18902–18908 (1999).
Conklin, B.R., Farfel, Z., Lustig, K.D., Julius, D. & Bourne, H.R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363, 274–276 (1993).
Miller, R.T., Masters, S.B., Sullivan, K.A., Beiderman, B. & Bourne, H.R. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature 334, 712–715 (1988).
Xu, W. et al. Functional role of the spatial proximity of Asp114(2.50) in TMH 2 and Asn332(7.49) in TMH 7 of the mu opioid receptor. FEBS Lett. 447, 318–324 (1999).
Ballesteros, J.A. et al. Activation of the beta(2)-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 (2001).
Moro, O., Lameh, J., Hogger, P. & Sadee, W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 (1993).
Strader, C.D. et al. Mutations that uncouple the beta-adrenergic receptor from Gs and increase agonist affinity. J. Biol. Chem. 262, 16439–16443 (1987); erratum 263, 3050 (1988).
Neve, K.A. et al. Modeling and mutational analysis of a putative sodium-binding pocket on the dopamine D-2 receptor. Mol. Pharmacol. 60, 373–381 (2001).
Urizar, E. et al. An activation switch in the rhodopsin family of G protein-coupled receptors: the thyrotropin receptor. J. Biol. Chem. 280, 17135–17141 (2005).
Simpson, M.M. et al. Dopamine D4/D2 receptor selectivity is determined by a divergent aromatic microdomain contained within the second, third, and seventh membrane-spanning segments. Mol. Pharmacol. 56, 1116–1126 (1999).
Guo, W. et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 27, 2293–2304 (2008).
Wilson, J., Lin, H., Fu, D., Javitch, J.A. & Strange, P.G. Mechanisms of inverse agonism of antipsychotic drugs at the D2 dopamine receptor: use of a mutant D2 dopamine receptor that adopts the activated conformation. J. Neurochem. 77, 493–504 (2001).
Guo, W., Shi, L., Filizola, M., Weinstein, H. & Javitch, J.A. From the cover: crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc. Natl. Acad. Sci. USA 102, 17495–17500 (2005).
Kota, P., Reeves, P.J., RajBhandary, U.L. & Khorana, H.G. Opsin is present as dimers in COS1 cells: identification of amino acids at the dimeric interface. Proc. Natl. Acad. Sci. USA 103, 3054–3059 (2006).
Goudet, C. et al. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J. Biol. Chem. 280, 24380–24385 (2005).
Hlavackova, V. et al. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 24, 499–509 (2005).
Kniazeff, J. et al. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat. Struct. Mol. Biol. 11, 706–713 (2004).
Kaupmann, K. et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
Damian, M., Martin, A., Mesnier, D., Pin, J.P. & Baneres, J.L. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 25, 5693–5702 (2006).
Springael, J.Y., Urizar, E., Costagliola, S., Vassart, G. & Parmentier, M. Allosteric properties of G protein-coupled receptor oligomers. Pharmacol. Ther. 115, 410–418 (2007).
Springael, J.Y. et al. Allosteric modulation of binding properties between units of chemokine receptor homo- and hetero-oligomers. Mol. Pharmacol. 69, 1652–1661 (2006).
Urban, J.D. et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 (2007).
Javitch, J.A. et al. The fourth transmembrane segment of the dopamine D2 receptor: accessibility in the binding-site crevice and position in the transmembrane bundle. Biochemistry 39, 12190–12199 (2000).
Costagliola, S. et al. Structure-function relationships of two loss-of-function mutations of the thyrotropin receptor gene. Thyroid 9, 995–1000 (1999).
Brini, M. et al. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). J. Biol. Chem. 270, 9896–9903 (1995).
Niv, M.Y., Skrabanek, L., Filizola, M. & Weinstein, H. Modeling activated states of GPCRs: the rhodopsin template. J. Comput. Aided Mol. Des. 20, 437–448 (2006).
van Dijk, A.D. et al. Data-driven: HADDOCK's adventures in CAPRI. Proteins 60, 232–238 (2005).
van Dijk, M., van Dijk, A.D., Hsu, V., Boelens, R. & Bonvin, A.M. Information-driven protein-DNA docking using HADDOCK: it is a matter of flexibility. Nucleic Acids Res. 34, 3317–3325 (2006).
Scheerer, P. et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 (2008).
Acknowledgements
We thank C. Galás for discussion and comments on the manuscript. Plasmids encoding apo-aequorin were a gift from V. Dupriez (Euroscreen). This work was supported in part by US National Institutes of Health grants DA022413 and MH054137 (to J.A.J.) and DA012923 (to H.W.), by the Lieber Center for Schizophrenia Research and Treatment, and by a European Molecular Biology Organization long-term fellowship (to E.U.). Computational resources of the David A. Cofrin Center for Biomedical Information (Institute for Computational Biomedicine, Weill Cornell Medical College of Cornell University) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Y.H. created all the mutant constructs and cell lines, helped to design the experiments, carried out the experimental assays and analyzed the results. I.S.M. performed the computational analysis. E.U., H.W. and J.A.J. helped to design experiments and interpret results. All the authors participated in the writing and editing of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 1033 kb)
Rights and permissions
About this article
Cite this article
Han, Y., Moreira, I., Urizar, E. et al. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5, 688–695 (2009). https://doi.org/10.1038/nchembio.199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.199
This article is cited by
-
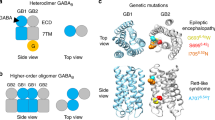
Biased signaling due to oligomerization of the G protein-coupled platelet-activating factor receptor
Nature Communications (2022)
-
Regulation of antral follicular growth by an interplay between gonadotropins and their receptors
Journal of Assisted Reproduction and Genetics (2022)
-
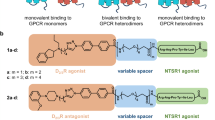
Bivalent ligands promote endosomal trafficking of the dopamine D3 receptor-neurotensin receptor 1 heterodimer
Communications Biology (2021)
-
Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation
Molecular Psychiatry (2020)
-
Minute-scale persistence of a GPCR conformation state triggered by non-cognate G protein interactions primes signaling
Nature Communications (2019)