Abstract
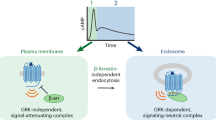
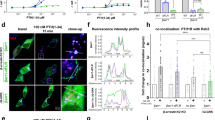
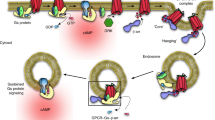
The generation of cAMP by G protein–coupled receptors (GPCRs) and its termination are currently thought to occur exclusively at the plasma membrane of cells. Under existing models of receptor regulation, this signal is primarily restricted by desensitization of the receptors through their binding to β-arrestins. However, this paradigm is not consistent with recent observations that the parathyroid hormone receptor type 1 (PTHR) continues to stimulate cAMP production even after receptor internalization, as β-arrestins are known to rapidly bind and internalize activated PTHR. Here we show that binding to β-arrestin1 prolongs rather than terminates the generation of cAMP by PTHR, and that cAMP generation correlates with the persistence of arrestin–receptor complexes on endosomes. PTHR signaling is instead turned off by the retromer complex, which regulates the movement of internalized receptor from endosomes to the Golgi apparatus. Thus, binding by the retromer complex regulates the sustained generation of cAMP triggered by an internalized GPCR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Perry, S.J. et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298, 834–836 (2002).
Premont, R.T. & Gainetdinov, R.R. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 69, 511–534 (2007).
Hanyaloglu, A.C. & von Zastrow, M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 (2008).
Ferrandon, S. et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 (2009).
Okazaki, M. et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. USA 105, 16525–16530 (2008).
Robben, J.H. et al. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc. Natl. Acad. Sci. USA 106, 12195–12200 (2009).
Calebiro, D. et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7, e1000172 (2009).
Finkelstein, J.S. & Arnold, A.L. Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. J. Clin. Endocrinol. Metab. 84, 1214–1219 (1999).
Neer, R.M. et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).
Horwitz, M.J. et al. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1–36) [hPTHrP-(1–36)] versus hPTH-(1–34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J. Clin. Endocrinol. Metab. 88, 1603–1609 (2003).
Vilardaga, J.P. et al. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J. Biol. Chem. 277, 8121–8129 (2002).
Castro, M. et al. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology 143, 3854–3865 (2002).
Ferrari, S.L., Behar, V., Chorev, M., Rosenblatt, M. & Bisello, A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J. Biol. Chem. 274, 29968–29975 (1999).
Malecz, N., Bambino, T., Bencsik, M. & Nissenson, R.A. Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol. Endocrinol. 12, 1846–1856 (1998).
Rosenblatt, M. When two keys fit one lock, surprises follow. Nat. Chem. Biol. 5, 707–708 (2009).
Nikolaev, V.O., Bunemann, M., Hein, L., Hannawacker, A. & Lohse, M.J. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 279, 37215–37218 (2004).
Nikolaev, V.O., Hoffmann, C., Bunemann, M., Lohse, M.J. & Vilardaga, J.P. Molecular basis of partial agonism at the neurotransmitter alpha2A-adrenergic receptor and Gi-protein heterotrimer. J. Biol. Chem. 281, 24506–24511 (2006).
Pitcher, J., Lohse, M.J., Codina, J., Caron, M.G. & Lefkowitz, R.J. Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry 31, 3193–3197 (1992).
Violin, J.D. et al. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 283, 2949–2961 (2008).
Lohse, M.J., Benovic, J.L., Codina, J., Caron, M.G. & Lefkowitz, R.J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 248, 1547–1550 (1990).
Lohse, M.J. et al. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J. Biol. Chem. 267, 8558–8564 (1992).
Keyel, P.A. et al. The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis. Mol. Biol. Cell 19, 5309–5326 (2008).
Burtey, A. et al. The conserved isoleucine-valine-phenylalanine motif couples activation state and endocytic functions of beta-arrestins. Traffic 8, 914–931 (2007).
Feinstein, T.N. & Linstedt, A.D. GRASP55 regulates Golgi ribbon formation. Mol. Biol. Cell 19, 2696–2707 (2008).
Garrido, J.L., Wheeler, D., Vega, L.L., Friedman, P.A. & Romero, G. Role of phospholipase D in parathyroid hormone type 1 receptor signaling and trafficking. Mol. Endocrinol. 23, 2048–2059 (2009).
Collins, B.M. et al. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 9, 366–379 (2008).
Collins, B.M. The structure and function of the retromer protein complex. Traffic 9, 1811–1822 (2008).
Bonifacino, J.S. & Rojas, R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7, 568–579 (2006).
Zucker, R.M. & Lerner, J.M. Wavelength and alignment tests for confocal spectral imaging systems. Microsc. Res. Tech. 68, 307–319 (2005).
Lerner, J.M. & Zucker, R.M. Calibration and validation of confocal spectral imaging systems. Cytometry A 62, 8–34 (2004).
Popoff, V. et al. The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 120, 2022–2031 (2007).
Gaidarov, I., Krupnick, J.G., Falck, J.R., Benovic, J.L. & Keen, J.H. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 18, 871–881 (1999).
Chauvin, S., Bencsik, M., Bambino, T. & Nissenson, R.A. Parathyroid hormone receptor recycling: role of receptor dephosphorylation and beta-arrestin. Mol. Endocrinol. 16, 2720–2732 (2002).
Sneddon, W.B. et al. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50). J. Biol. Chem. 278, 43787–43796 (2003).
Baillie, G.S. et al. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. USA 100, 940–945 (2003).
Hoffmann, R., Baillie, G.S., MacKenzie, S.J., Yarwood, S.J. & Houslay, M.D. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 18, 893–903 (1999).
Pippig, S. et al. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J. Biol. Chem. 268, 3201–3208 (1993).
Krupnick, J.G., Gurevich, V.V. & Benovic, J.L. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J. Biol. Chem. 272, 18125–18131 (1997).
Mahon, M.J., Bonacci, T.M., Divieti, P. & Smrcka, A.V. A docking site for G protein βγ subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol. Endocrinol. 20, 136–146 (2006).
Johnston, C.A., Kimple, A.J., Giguere, P.M. & Siderovski, D.P. Structure of the parathyroid hormone receptor C terminus bound to the G-protein dimer Gbeta1gamma2. Structure 16, 1086–1094 (2008).
Yang, M., He, R.L., Benovic, J.L. & Ye, R.D. β-Arrestin1 interacts with the G-protein subunits β1γ2 and promotes β1γ2-dependent Akt signalling for NF-κB activation. Biochem. J. 417, 287–296 (2009).
Shi, H., Rojas, R., Bonifacino, J.S. & Hurley, J.H. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat. Struct. Mol. Biol. 13, 540–548 (2006).
Aubry, L., Guetta, D. & Klein, G. The arrestin fold: variations on a theme. Curr. Genomics 10, 133–142 (2009).
Nothwehr, S.F., Bruinsma, P. & Strawn, L.A. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol. Biol. Cell 10, 875–890 (1999).
Hierro, A. et al. Functional architecture of the retromer cargo-recognition complex. Nature 449, 1063–1067 (2007).
Seaman, M.N. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 120, 2378–2389 (2007).
Arighi, C.N., Hartnell, L.M., Aguilar, R.C., Haft, C.R. & Bonifacino, J.S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123–133 (2004).
Eaton, S. Retromer retrieves wntless. Dev. Cell 14, 4–6 (2008).
Vergés, M. et al. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 6, 763–769 (2004).
Acknowledgements
This work was supported by the US National Institutes of Health award R01DK087688 (to J.-P.V.). We thank J. Bonifacino for plasmids encoding retromer subunits Vps26 and Vps29YFP and L. Traub for plasmids encoding β-arrestin1tom and β-arrestin1[I386A, V387A]tom.
Author information
Authors and Affiliations
Contributions
T.N.F. performed most of the experiments with the support of V.L.W., J.A.A., D.S.W., S.F. and T.J.G.; J.-P.V. designed and supervised the experiments; J.-P.V. and T.N.F. analyzed the data and wrote the manuscript; all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–10 (PDF 1653 kb)
Supplementary Video 1
A three-dimensional reconstruction of a representative endosome labeled with Vps29YFP (blue), GFPPTHR (green) and β-arr1tom (red). (MOV 13562 kb)
Rights and permissions
About this article
Cite this article
Feinstein, T., Wehbi, V., Ardura, J. et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol 7, 278–284 (2011). https://doi.org/10.1038/nchembio.545
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.545
This article is cited by
-
Structural details of a Class B GPCR-arrestin complex revealed by genetically encoded crosslinkers in living cells
Nature Communications (2023)
-
β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR
Nature Chemical Biology (2023)
-
Precise druggability of the PTH type 1 receptor
Nature Chemical Biology (2022)
-
Parkinson's in the bone
Cell & Bioscience (2021)
-
Compartmentalized GPCR Signaling from Intracellular Membranes
The Journal of Membrane Biology (2021)