Abstract
Although emerging roles of protease-activated receptor1&2 (PAR1&2) in cancer are recognized, their underlying signalling events are poorly understood. Here we show signal-binding motifs in PAR1&2 that are critical for breast cancer growth. This occurs via the association of the pleckstrin homology (PH) domain with Akt/PKB as a key signalling event of PARs. Other PH-domain signal-proteins such as Etk/Bmx and Vav3 also associate with PAR1 and PAR2 through their PH domains. PAR1 and PAR2 bind with priority to Etk/Bmx. A point mutation in PAR2, H349A, but not in R352A, abrogates PH-protein association and is sufficient to markedly reduce PAR2-instigated breast tumour growth in vivo and placental extravillous trophoblast (EVT) invasion in vitro. Similarly, the PAR1 mutant hPar1-7A, which is unable to bind the PH domain, reduces mammary tumours and EVT invasion, endowing these motifs with physiological significance and underscoring the importance of these previously unknown PAR1 and PAR2 PH-domain-binding motifs in both pathological and physiological invasion processes.
Similar content being viewed by others
Introduction
G protein-coupled receptors (GPCRs) are the largest family in mammalian cells, mediating a plethora of physiological responses1,2. Despite the fact that GPCRs emerge as oncogenes that regulate cancer-associated signalling networks, their role in tumour biology is not well understood. Indeed, large-scale genome analyses of multiple human tumours have uncovered novel GPCR alterations, as well as aberrant overexpression of GPCRs in cancer3,4. It is imperative to determine which of the GPCRs are cancer instigators rather than bystanders to enable identification of candidate genes for future targeted personalized medicine.
During cancer progression, normal epithelial organization is disrupted and cells are maintained outside their normal niches5,6. Both soluble and matrix-immobilized proteases are present in the dynamic and flexible microenvironment of a tumour and contribute to the process of cancer advancement7. One example is the activation of cell surface protease-activated receptors (PARs). Mammalian PARs are a subgroup of GPCRs that form a four-member family8,9,10,11,12. PAR1 and PAR2 play a central role in epithelial tumour growth in a variety of malignancies13,14,15,16. Whereas PAR2 is not considered a thrombin receptor (unlike PAR1,3 and4), the PAR1-tethered ligand SFLLRN is capable of transactivating PAR2 (refs 17, 18). Increasing evidence supports the notion that PAR1 and PAR2 exist in close proximity and act as one functional unit forming heterodimers17,18,19. Consistently, we have found that PAR2 plays a dominant role in PAR1–PAR2-instigated tumour activity20.
Among the protein modules that drive intermolecular interactions in cellular signalling, the pleckstrin homology (PH) domain is most common. PH domains are mainly recognized by their structural characteristics, and with a seven-stranded β-sandwich and a C-terminal α-helix21. While PH domains lack primary sequence similarity, their superfold assembly represents a particularly stable structural scaffold employed in many different functions22.
Here we describe PH-domain-binding motifs in PAR1 and PAR2 C-tails that are necessary for PAR-driven tumour growth and time-limited placental trophoblast invasion. We propose that these PH-domain-binding motifs may serve as an important molecular mechanism within the PAR signalling network and provide a platform for future drug therapy design.
Results
PAR2 associates with Akt/PKB via its PH domain
To identify a key signalling partner that plays a role in PAR2-driven tumour growth (Supplementary Fig. 1), we analysed the interaction between PAR2 and Akt/PKB, a serine/threonine protein kinase that plays a pivotal role in tumour cell survival, proliferation and invasiveness23,24. HEK-293T cells were transiently transfected with hPar2 and cell lysates, before and after SLIGKV-PAR2 activation, were immunoprecipitated with anti-PAR2 antibodies and analysed for Akt/PKB co-association. A tight association was observed after 2–10 min of activation, which declined thereafter (Fig. 1a,b). This interaction takes place via the binding of PAR2 C-tail with the PH domain of Akt/PKB, as evaluated by the GST-PAR2 C-tail pull-down assay (Fig. 1c). Akt/PKB also co-associates with PAR1 via its PH domain (Supplementary Fig. 2).
(a) Schematic presentation of Akt/PKB. (b) Immunoprecipitation (IP) analysis of PAR2 and Akt/PKB. HEK 293T cells were transfected with wt hPar2. IP was performed following PAR2 activation using anti PAR2 antibodies and immunoblotting with anti-Akt antibodies. Co-immunoprecipitation (Co-IP) was performed following SLIGKV PAR2 activation of wt hPar2 at 2–10 min. (c) GST-PAR2-C-tail binds wt Akt or Akt-PH-domain module alone. HU nearly normal cells (naive cells not expresing endogenous PAR2) were transiently transfected or not with either GFP-wt Akt or GFP-PH-domain alone. Cell lysates were applied to the GST-PAR2 C-tail. Specific binding was seen following separation on SDS–PAGE and detection using anti-GFP antibodies. (d) PAR2 mutant H349A fails to associate with Akt. HU cells were transiently transfected either with wt hPar2, PAR2 mutant R352A or PAR2 mutant H349A. Cell lysates were immunoprecipitated following SLIGKV PAR2 activation using anti-PAR2 antibodies. Detection by western blot analyses of Akt PAR2 association was performed using anti-Akt antibodies. Phosphorylation of Akt was detected using anti-phospho Ser473 antibodies. Where indicated, IP detection of PAR2 was carried out using anti PAR2 SAM11 ab (1 μg per assay) at 1:500 dilution. (e) PAR2 mutant H349A fails to associate with GST-Akt-PH domain. Application of HU cells following transient transfection with the indicated constructs (for example, wt hPar2, a short hPar2 C-tail-K356Z and the hPar2 mutant H349A) on columns of GST-Akt-PH domain, resulted in effective association as detected by western blot analysis with the wt and PAR2-K356Z constructs. No binding was seen in the presence of the PAR2 point mutation H349A. (f) Expression of PAR2 constructs. HU cells were transiently transfected with the indicated PAR2 constructs. Level of construct expression is detected by Western blot analysis, using anti PAR2 antibodies. (g) Delta PH-Akt does not associate with PAR2 C-tail. HU cells were transiently transfected with YFP-hPar2 construct and either GFP-wt-Akt or myr-Akt delta 4–129 (PH domain) HA-tag. IP was performed following PAR2 SLIGKV activation, using anti-PAR2 antibodies and immunoblotting with anti-Akt antibodies. While co-association was observed in cells transfected with GFP-wt Akt, in-contrast, no association was seen following SLIGKV PAR2 activation in cells transfected with the myr-Akt delta 4–129 (PH-domain) HA-tag plasmid. Control for protein loading is shown by levels of β-actin.
To conclusively identify the critical amino acids involved in this association, point mutations were inserted in the PAR2 C-tail downstream of the membrane-anchoring site (R352A and H349A, Supplementary Table 1). While binding was observed with mutant R352A similar to wt PAR2, no association was seen with H349A (Fig. 1d). The PAR2-bound Akt was functionally active, as demonstrated by high phosphorylation levels of Ser473 (Fig. 1d). Importantly, introduction of these mutants did not alter the cell distribution of PAR2, as shown by the analysis of the cell surface expression, although truncated hPar2 was expressed to somewhat a lower level (Supplementary Fig. 3). When cell lysates overexpressing either wt hPar2 or a short deleted PAR2 C-tail construct (hPar2-K356Z) and mutant hPar2-H349A were applied to GST-PH-Akt columns, a potent binding association with wt hPar2 and with hPar2 K356Z was obtained. No binding was observed when cell lysates expressing mutant PAR2 H349A were applied, or when wt hPar2 was applied to GST beads alone (Fig. 1e). Levels of the various constructs expression are shown in Fig. 1f. When an Akt construct that was devoid of the PH domain was utilized, no association between PAR2 and Akt took place (Fig. 1g). This provides support for the role of the PH domain of Akt in the binding association between PAR2 and Akt. Furthermore, the mutant hPar2-H349A effectively inhibited cell migration and wound closure in a similar way to truncated hPar2. In contrast, activation of wt hPar2 potently induced migration and closure of the wound by 72 h. SLIGKV activation of mutant hPar2-R352A induced migration, although at a slower rate compared with wt hPar2 (Supplementary Fig. 4).
Other PH-domain signal proteins associate with PAR1/2
We next examined the possibility that additional signal proteins carrying a PH domain are capable of association with PAR. ExPASy proteomics was used to identify a wide panel of PH-domain-containing proteins. Among others, the signal proteins Etk/Bmx, Akt, Vav, SOS1 and GAB1 were found to carry this domain. We chose to focus on two signalling proteins, Etk/Bmx and Vav3. The interaction between PAR2 and Etk/Bmx, a member of the nonreceptor tyrosine kinase family encoded by the BMX gene25,26 was examined. We previously examined the interaction between Etk/Bmx and PAR1 (ref. 27). Distinct association between PAR2 and Etk/Bmx was observed at 15–20 min, which declined thereafter (Fig. 2a–c). In contrast, no binding was obtained when a truncated form of hPar2, devoid of the entire cytoplasmic tail, was ectopically expressed in the cells. This association takes place via the binding of the PAR2 C-tail with the PH domain of Etk/Bmx, as evaluated by the GST-PH-Etk/Bmx pull-down assay (Fig. 2d).
(a) Schematic presentation of wt and truncated PAR2. (b) Scheme of Etk/Bmx. (c) HEK293T cells were transfected with T7 Etk/Bmx and either wt hPar2 or a truncated form of hPar2 plasmids. IP was performed following PAR2 activation using anti-PAR2 antibodies and immunoblotting with anti-T7 for Etk/Bmx detection (T7 tagged Etk/Bmx) of a Western blot. Effective co-immunoprecipitation was seen following SLIGKV PAR2 activation of wt hPar2 but not with a truncated form of hPar2. (d) PAR2 binding to GST-PH-Etk/bmx domain. Cell lysates expressing PAR2 were applied on either GST-PH-Etk/Bmx or GST beads alone, specific binding was seen on the GST-PH-Etk/Bmx beads but not on the GST beads, as detected by Western blot analyses. (e) Co-IP between wt PAR2 and PAR2 deleted constructs. HEK293T cells were transiently transfected with the various PAR2 constructs. While effective association was seen following SLIGKV PAR2 activation in the wt PAR2 and the shortest C-tail PAR2 K356Z deleted construct, no association was observed in the presence of the truncated form of PAR2, which was devoid of the entire C-tail. (f) Schematic representation of PAR2 C-tail deleted constructs. (g) PAR2 mutant H349A fails to bind Etk/Bmx. HU cells were transiently transfected with either wt hPar2, PAR2 mutant R352A, or PAR2 mutant H349A. Cell lysates were immunoprecipitated following SLIGKV PAR2 activation with anti-PAR2 antibodies. Detection for Etk/Bmx PAR2 association was carried out by anti-T7 antibodies. Tyr-phosphorylation of bound Etk/Bmx was detected using anti-4G10 antibodies for the detection of phosphotyrosine residues. Levels of PAR2 are shown as control. When indicated, detection of PAR2 was carried out using either SAM 11ab (Santa Cruz Biotechnology) at1:500 dilution.
We then determined the minimal PH-domain-binding region within the PAR2 C-tail sequence. For this purpose, we prepared deleted PAR2 C-tail constructs. Effective co-association with the Etk/Bmx PH domain was observed with the shortest C-tail construct hPar2-K356Z (Fig. 2e,f), and similar with the wt hPar2 construct. Following insertion of mutations into the short K356Z region, we found that in HEK 293T cells overexpressing either R352A or H349A mutants no association was seen with the mutant H349A; however, the R352A mutant did associate with the Etk/Bmx PH domain (Fig. 2g and supplementary Fig. 5A). PAR2 C-tail-bound Etk/Bmx was functionally active, allowing downstream signal association, as demonstrated by induced Tyr-phosphorylation levels (Fig. 2g). We therefore conclude that the amino acid histidine at position 349 is critical for the association of PAR2 with the Etk/Bmx PH domain, as was seen above with Akt/PKB.
We observed that, while Akt was abundantly expressed in the cancer cell lines examined, Etk/Bmx expression was restricted to CL1, a prostate cancer cell line (Fig. 3a). Lysates of cells expressing endogenous or transfected Etk/Bmx, as well as lysates of cells that do not express Etk/Bmx, were loaded on glutathione S-transferase beads fused to the individual PAR C-tails (either PAR1 or PAR2) for a pull-down assay. Specific association of Etk/Bmx with both GST-PAR1 and GST-PAR2 C-tails was observed (Fig. 3b). In contrast, in HCT-116 cells that lack endogenous Etk/Bmx, effective association with Akt was observed for both PAR1 and PAR2 C-tails (Fig. 3b).
(a) Western blot analysis of Akt and Etk/Bmx in cell lines. Cell lysates of the indicated cell lines were analysed by western blot analysis. Application of anti-Etk/Bmx or anti-Akt antibodies showed that endogenous Etk/Bmx was present only in CL1 cells, an aggressive prostate cancer cell line, or following ectopic overexpression in HEK 293T cells. In contrast, Akt was abundantly present in all the cell lines analysed. (b) PAR1- and PAR2-GST-C-tails associate with Etk/Bmx and Akt. Specific association of the Akt-PH domain was seen in cells that do not express Etk/Bmx (for example, HEK 293T and HCT116 cells). HEK293T cell lysates overexpressing etk/bmx plasmid concentrations (0.5–2 μg) showed no association with the Akt-PH domain. Application of HEK293T cells transfected to overexpress Etk/Bmx showed effective immobilization on both PAR1 and PAR2 C-tails. Application of HCT116 colon cancer cells that do not express Etk/Bmx showed a marked association with Akt. (c) PAR2 binds GST-PH-Akt. Specific association of the Akt-PH domain was seen in cells that do not express Etk/Bmx (for example, HEK 293T and HCT116 cells). HEK293T cell lysates overexpressing etk/bmx plasmid concentrations (0.5–2 μg) showed no association with the Akt-PH domain. (d) Western blot analysis. Levels of T7-Etk/Bmx following increased etk/bmx plasmid transfection are shown. β-actin was used as a loading control. (e) PAR2 binds GST-PH-Vav3. Specific association of the Vav3-PH domain was seen in cells without Etk/Bmx expression (for example, HEK 293T and HCT116 cells). HEK293T cell lysates overexpressing etk/bmx plasmid concentrations (0.5–2 μg) showed no association with the Vav3-PH domain. When indicated, detection of PAR2 was carried out using either SAM 11ab (Santa Cruz Biotechnology) at 1:500 dilution. (f) Alignment PH-domain sequences of Etk/Bmx, Vav3 and Akt. (d) PAR2 binds GST-PH-Vav3. Multiple sequence alignment of PH-Etk/Bmx, PH-Vav3 and PH-Akt was performed using T-coffee (available at http://www.ebi.ac.uk/Tools/msa/tcoffee).
Hierarchy of binding
We also examined the priority of binding for Etk/Bmx by adding appropriately modified cell lysates to GST beads fused either to PH-Akt (Fig. 3c,d) or to PH-Vav3 columns (Fig. 3E). Vav3 oncogene, a guanine nucleotide exchange factor (GEF) of the Rho family GTPases, belongs to the Vav protein family28,29,30 and is ubiquitously expressed in breast and prostate cancers. Although all Vav family proteins have similar structural features, they display different tissue expression patterns. Vav1 is primarily expressed in haematopoietic lineages, while Vav2 is ubiquitously expressed. Vav3 has a broad but distinct expression profile compared with that of Vav2. We showed binding with Vav3 by a pull-down assay using PH-Vav3 columns (Fig. 3e and Supplementary Fig 5B). Before the GST-binding assay, the applied lysates of tumour cell lines were analysed for endogenous expression of either Akt or Etk/Bmx or both. These experiments showed that PAR1 and PAR2 have priority for binding with the Etk/Bmx PH domain over Akt or Vav3. Only when Etk/Bmx was absent did another PH-domain signal protein such as Akt bind with both PAR1 and PAR2 C-tails.
PH-Akt/Vav3/Etk/Bmx bind to the same site on the PAR2 C-tail
HEK293T cells overexpressing PAR2 mutants (R352A and H349A) or PAR2 deletion constructs were added to GST-PH-Etk/Bmx, GST-PH-Akt or GST-PH-Vav3. While a specific association was observed with wt hPar2, hPar2 K368Z and hPar2 K356Z, as well as the mutant R352A, no binding was seen when H349A was added to GST-PH-Etk/Bmx, GST-PH-Akt or GST-PH-Vav3 (Fig. 3a–d). Histidine at position 349 is thus a critical amino acid for PH-signal proteins. Similarly, the same binding region in the PAR1 C-tail was found for the PH-domain signal proteins tested27. Sequence alignment of PH-Etk/Bmx, PH-Vav3 and PH-Akt showed a high number of different primary sequences (Fig. 3f).
Characterization of PH-Akt/Etk/Bmx binding
There have been a considerable number of reports about the proposed function of Akt PH domain lipid membrane recruitment and AKT activation31. AKT is a known phospholipid-binding serine/threonine kinase, and a key component of the phosphoinositide 3-kinase (PI3K) cell survival-signalling pathway that is aberrantly activated in many human cancers32. We observed distinct binding of either wt Akt or the GFP-Akt PH domain module alone with the PAR2 C-tail. In contrast, the Akt PH domain mutant R25C, which has low lipid-binding affinity, failed to associate with PAR2 (Fig. 4a). In addition, the application of LY294002, a PI3K inhibitor, completely abrogated the otherwise potent association between PAR2 and Akt (Fig. 4b). The involvement of phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3; PIP3) in the PAR2–Akt association is supported by the potent inhibition of PAR2-Akt binding in the presence of Ins (1,3,4,5) P4 (IP4) (Supplementary Fig. 6). We conclude that the Akt–PAR2 association involves membrane anchoring via the PH domain. On the other hand, both the PH domain mutant R28C of the Etk/Bmx PH domain, which is incapable of lipid binding, and the GFP-Etk/Bmx PH domain module alone, exhibited potent binding with PAR2 C-tail (Fig. 4c).
(a) PH-Akt module binds PAR2-C-tail but not mutant R25C. HU cells were transiently transfected with hPar2 construct and either with GFP-wt-Akt, GFP-PH-Akt domain alone or GFP-R25C. Immunoprecipitation of cell lysates following PAR2 activation was carried out using anti-PAR2 antibodies. Detection of either wt Akt or Akt-PH domain alone associated with PAR2 was performed with anti-GFP. While wt GFP-Akt and GFP-PH domain alone were shown to bind PAR2, no binding was obtained when the mutant R25C of low lipid-binding-affinity was present. (b) PI3K inhibitor (LY 294002) inhibits binding of Akt to PAR2. Treatment of HU cells with increasing concentrations of LY294002 following transient transfection with hPar2 and SLIGKV activation was performed. Cell lysates were immunoprecipitated with anti-PAR2 antibodies, and anti-Akt was used to assess the association of Akt with the PAR2 C-tail. A potent inhibition of Akt-PAR2 association was observed in the presence of LY294002. (c) GFP-PH-Etk/Bmx module and mutant R28C bind PAR2. HU cells were transiently transfected with hPar2 construct and with either GFP-PH-Etk/Bmx domain alone or GFP-R28C Etk/Bmx mutant with low lipid-binding affinity. Immunoprecipitation of cell lysates following PAR2 activation was performed using anti-PAR2 antibodies. Anti-GFP was used to detect the intact Etk/Bmx-PH domain alone associated with PAR2 and the mutant R28C. Both constructs bind PAR2 potently. (d) Co-IP between wt and Etk/Bmx modulation constructs. Co-IP analyses performed utilizing HU cells transiently transfected with either wt hPar2, wt Etk/Bmx, Myr-dPH construct or TH-SH2-SH3-KD. No association between Etk/Bmx and PAR2 was observed either with Myr-dPH constructs or TH-SH2-SH3-KD construct deleted for the PH domain. In comparison, effective association was obtained with wt Etk/Bmx. (e) LY294002 does not inhibit association of Etk/Bmx with PAR2. Treatment of HU cells with increasing concentrations of LY294002 following transient transfection with both T7-etk/bmx and hPar2 and SLIGKV activation was performed. Cell lysates were immunoprecipitated with anti-PAR2 antibodies and Etk/Bmx- PAR2 association byanti-T7.
To better characterize the nature of binding between PAR2 and the Etk/Bmx PH domain, analyses of additional Etk/Bmx constructs were carried out. These constructs were an Etk/Bmx construct whose PH domain was replaced by a myristoyl group attached to the N-terminal portion, Myr-dPH, and a construct depleted of the PH-domain containing the kinase domain, SH3, and SH2 domains, TH-SH2-SH3-KD (refs 33, 34). On SLIGKV activation of PAR2, no association between Myr-dPH Etk/Bmx and PAR2 was observed (Fig. 4d). Similarly, no interaction was seen when a deleted PH domain TH-SH2-SH3-KD construct was used (Fig. 4d). Accordingly, the presence of LY294002 PI3K inhibitor did not abrogate the association between PAR2 and Etk/Bmx (Fig. 4e). Hence, the Etk/Bmx PH domain association is completely lipid-independent and binds via a protein–protein interaction. This stands in contrast to Akt PH domain association with PAR2 C-tail, which is membrane lipid-dependent.
PAR2 PH-domain-binding site is critical for EVT invasion
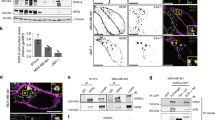
Establishment of human pregnancy involves the well-orchestrated invasion to the uterus wall of specialized cells termed extravillous trophoblasts (EVT). We have utilized an in vitro model based on isolation of villi from early gestation human placentae plated on Matrigel20,35,36,37. Placental specimens were harvested at the end of the first trimester (10–12 weeks) and those expressing little or no endogenous PAR1 and PAR2 were selected. These cultures were transfected with lentiviral wt hPar1, viral hPar1-7A mutant, wt hPar2, or hPar2 mutant H349A, and were activated by their respective ligands. The impact of PAR1 or PAR2 activation on wt and mutant forms was evaluated by comparing the depth of invasion. EVT invasion into the Matrigel reached a maximal invasion depth of 100 μm, as evaluated by 5-μm serial sections of the Matrigel cast. Histological analyses of haematoxylin and eosin (H&E) staining are shown in Fig. 5a–h. Significant inhibition in EVT cell invasion was seen in the presence of the mutants that are incapable of association with PH-domain signal protein/s (activated hPar1-7A or hPar2 H349A, Fig. 5a, lanes d and h, respectively), and before activation (Fig. 5a, b and e). In contrast, a high level of invasion was observed following either TFLLRN PAR1 or SLIGKV PAR2 activation, with invasion reaching up to 100 μm. Highly proliferative EVT cells at the tip of the villi recapitulate outgrowth and depth of invasion. Nuclear staining of ki67 demonstrates the extent of proliferating cells following wt hPar2 and the mutant forms following activation (Fig. 5i–l). The number of cells per high-power field for each treatment was evaluated at an equal level of invasion (60 μm), as shown in the representative histogram (Fig. 5m–n).
(a–h) Morphology of EVT column growth. Placental explants from the EVT–Matrigel cylinder cast after H&E staining (magnification × 40). Serial 5-μm sections of the Matrigel cylinder were embedded in paraffin blocks prepared for each of the treatment. Images of representative H&E-stained sections at 60 μm depth are presented. (a) Untreated (control), (b) wt hPar1-infected explants, (c) thrombin (1 U ml−1) activation, (d) hPar1 7A mutant and thrombin activation, (e) control, untreated, (f) wt hPar2, (g) wt hPar2 and SLIKGV (100 μM) activation, (h) hPar2 mutant H349A and SLIKGV (100 μM) activation. Note the increase in EVT-migrating cells and cell mass at the tip of the villi following either PAR1 or PAR2 activation. In contrast, a blunt end of the villi without sprouting cells was seen at the villi tip in the presence of mutants following activation treatments. The experiment was terminated 72 h after EVT treatment. Each experiment was performed using three different placentae, in triplicate. (i–l) Immunohistochemical staining of Ki67. Ki67 levels (measure of proliferation) were evaluated in (i) untreated (control), (j) wt hPar2, (k) wt hPar2 and SLIKGV (100mM) activation and (l) hPar2 mutant H349A and SLIKGV (100mM) activation. (m) Quantification of EVT outgrowth. Cell number was determined at a 60-μm depth of invasion as shown by the histogram mean values. Cells (not the villous compartment) were counted per high-power field (HPF) and expressed as mean±s.e.m. Post hoc evaluation of multiple comparisons (ANOVA Tukey HSD) showed a P value of 0.0001 within groups. The mean difference was significant at the 0.05 level. ANOVA evaluation was performed with the IBM/SPSS 20.0 software (Chicago, IL, USA). (n) Quantification of Ki67. Histogram of the mean values of Ki67 nuclear staining is shown. Cell nuclei were counted per HPF and expressed as mean±s.e.m. Post hoc evaluation of multiple comparisons (ANOVA Tukey HSD) showed a P value of 0.0003 within groups. The mean difference was significant at the 0.07 level. ANOVA evaluation was performed with the IBM/SPSS 20.0 software.
PAR2 Histidine349 is critical for tumour growth in vivo
The physiological significance of the PAR2 PH domain motif is also demonstrated using a xenograft model of tumour growth (Fig. 6a–c). We generated stable clones of a nearly normal fibrocystic cell line HU that lacked expression of endogenous PAR2 but expressed a luciferase construct and one of the following plasmids: wt PAR2, a truncated PAR2, and PAR2 mutants; R352A; or mutant R349A (Fig. 6d). The stable clones were inoculated subcutaneously into nude mice and were analysed for tumour growth. While large and vascularized tumours developed in mice inoculated with wt PAR2, no to minimal change in tumour size was seen in mutant R352A, and markedly smaller tumours were observed with the PAR2 mutant H349A, compared with PAR2-truncated clones (Fig. 6a–c). As mentioned above, the PAR2 R352A mutant did not abrogate PAR2 association with Etk/Bmx, Akt and the PH domain of Vav3 (Fig. 3c,d). In contrast, mutant H349A effectively abrogated the PAR2 association with all PH-domain signal proteins tested; hence, His (H) at position 349 is a critical amino acid for binding to all proposed downstream PH-domain signal proteins. Approximately 20-fold larger tumours were observed in mice inoculated with wt hPar2 cells (825 mm3; P<0.0007) as compared with those in mice inoculated with control cells (40 mm3). In a Matrigel invasion assay, wt PAR2 consistently elicited a significantly higher level of HU cell invasion following PAR2 activation. In contrast, in the presence of PAR2 H349A, no Matrigel invasion was observed, comparable to levels in control mice, regardless of SLIGKV activation (Supplementary Fig. 7). Likewise, when a mutated form of hPar1 (hPar1-7A) incapable of associating with the PH domain of Etk/Bmx (hPar1-7A) was analysed in a murine xenograft mammary model in vivo, a dramatic reduction in the otherwise large tumours was observed (Supplementary Figs 8 and 9).
Stable clones of HU cells were prepared expressing luciferase and either wt hPar2, or a truncated hPar2 (devoid of the cytoplasmic tail), or PAR2 mutant R352A, or PAR2 mutant H349A. The various clones (3 × 106 cells) were inoculated subcutaneously in nude mice. (a) Representative bioluminescence images. Levels of tumour luciferase following various cell-clone inoculation treatments. (b) Tumour morphological appearance. At the end of the experiment (for example, within 31 days; I–V), mice were terminated and the tumours (I–V) were excised, measured and weighed. (c) Measurements of tumour volume. Tumours were weighed and measured for size at the indicated time points and tumour volume (mm3) was calculated. Error bars show s.d.; * P<0.005. Data shown are representative of three independent experiments. (d) Levels wt and modified PAR2 in the stable clones. Stable clones expressing the various hPar2 constructs, either wt hPar2 or truncated hPar2, hPar2 mutant R352A or mutant H349A, are shown using PCR. GAPDH levels were used as the control housekeeping gene.
It is interesting to note that neutrophil elastase (NE) is incapable of inducing the PAR2-PH-Etk/Bmx association (Supplementary Fig. 10), while association of PAR1 to either PH-Etk/Bmx or PH-Akt is elicited by NE (Supplementary Fig. 11).
Discussion
PH domains are conserved protein motifs present in diverse signal-transducing proteins. They are known to be versatile modules in protein–protein interaction platforms in a plethora of physiological events38,39. We describe binding motifs capable of selective association with PH domains of Etk/Bmx, AKT and Vav3 in the C-tails of mammalian PAR1 and PAR2. These binding motifs are necessary for tumour development as well as physiological placental EVT–uterus interactions. Mutations inserted to the PAR1 PH domain (Supplementary Figs 7 and 8) and point mutation at H349A in PAR2 markedly attenuated xenograft tumour growth in a murine model of cell migration (Supplementary Fig. 4) and of placental–trophoblast time-limited invasion.
Despite the fact that sequence identity between PH domains is limited, their tertiary structures are strikingly similar. Although little is known about the molecular basis of PH-domain function, several lines of evidence indicate that these domains are critical for receptor activity. For example, coupling of insulin receptor and insulin receptor substrate, IRS-1, depends in part on the IRS-1 PH domain40,41. The PH domain of β-adrenergic receptor kinase is required for its interaction with the βγ-subunits of heterotrimeric G-proteins42.
Whereas many studies have addressed PH-domain-phospholipid-binding properties, protein–protein interactions are only now being investigated43. The PH domain of oncogenic Dbl mediates targeting to the cytoskeletal matrix and was found to be necessary for oncogenic transformation44. The PH domains in guanine nucleotide-binding proteins are essential for C-terminal association with the Dbl homology (DH) RhoGEF catalyst. Furthermore, phosphoinositol-lipid-binding PH sites are effective modules for both small guanine nucleotide-binding proteins45,46 and Gα-subunits47. Thus, both lipid-binding capabilities and protein–protein interactions play roles in PH-domain module interactions.
AKT activation is driven by binding of the PH domain to PIP3 or PIP2 for membrane localization, followed by the phosphorylation of serine 473 and threonine 308 (ref. 48). In addition, the PH domain plays a critical regulatory role in AKT function, and mutations disrupting the PH-domain function are apparently important in physiological and disease processes. For example, in a drosophila model, PIP3 levels are lethal when PTEN is lacking. Rescue survival is possible only if the drosophila AKT PH domain is inactive and incapable of binding lipid49. This indicates that AKT is a critical target, activated by increased PIP3, for a second lipid messenger pathway. A point mutation introduced into the PH-domain lipid-binding pocket of AKT1, whereby arginine is replaced by cysteine at amino acid 25 (AKT1R25C), results in low-affinity AKT-phospholipid binding, with both inhibited recruitment of AKT to the membrane and association with PAR2 (ref. 50). In another lipid-binding pocket mutation of the AKT–PH domain, E17K alters interactions of the pocket and activates AKT1 by means of pathological localization to the plasma membrane51. This mechanism suggests a direct role for AKT1 in human cancer and adds to the known genetic alterations that promote oncogenesis through the phosphatidylinositol-3-OH kinase/AKT pathway. Our data demonstrate that PAR1 and PAR2 harness the AKT pathway via binding to their PH domains, thus enabling their downstream network signalling.
It is well established that PIP3 functions to activate AKT via binding to the PH-domain-mediating translocation to the plasma membrane for the appropriate downstream signalling. Ins (1,4,5)P3 (IP3) however can be converted to IP4 by a family of inositol trisphosphate kinases. Once generated, IP4 can act as a soluble analogue of PIP3 and thereby negatively regulates PIP3 AKT-PH signalling. Indeed, our data show that potent inhibition of the association between PAR2 and PH-Akt that requires PIP3 is observed in the presence of increased IP4 concentrations (Supplementary Fig. 6). This regulation is similar to that of other diverse critical decision processes downstream of many receptors. Specific examples are the negative regulation of neutrophil signalling and chemotaxis52, neutrophil survival and bacteria killing by neutrophils, of overall importance for neutrophil function and innate immunity53.
Naturally occurring mutations in the PH domain of Bruton’s tyrosine kinase interfere with phosphoinositide-binding properties and with X-linked agammaglobulinaemia in humans and X-linked immunodeficiency in mice54. Here we show that, in contrast to Akt PH domain lipid-binding dependency, the PH domain of Etk/Bmx binds PAR2 in a phospholipid-independent manner. This is based on the strong PAR2-Etk/Bmx association with the mutant that is incapable of binding lipids, and on the lack of association of modified Etk/Bmx constructs, in which the PH domain was replaced with a myristoyl group or with a depleted PH domain construct. This finding is in line with other Etk/Bmx protein–protein interactions, such as binding to the FAK-FERM domain55. It was similarly demonstrated that the PH domain of an Etk/Bmx lipid-binding-deficient mutant retained potent FAK-binding capability, indicating that the regulation and association of Etk/Bmx by FAK is independent of lipid-binding activity of the PH domain.
Vav3 proto-oncogene is a member of Vav belonging to the Dbl family of GEF for the Rho family of GTP-binding proteins. The characteristic structural domains of the family include an N-terminal calponin homology domain, an acidic region, a DH domain and a PH domain, among others. Generally, in these protein families the PH domain is located at the C-terminal DH helix and contributes to the support of GEF activity56,57. It is possible, but remains to be shown, that the preference for Etk/Bmx association with PARs over the Akt or Vav3 PH domain is due to direct protein–protein interactions, independent of lipid involvement, although the nature of the PH-Vav3 interaction remains to be fully determined.
Overall, regardless of unusually different primary sequences, PH domains share a conserved fold made up of a b-barrel composed of two roughly perpendicular, antiparallel beta-sheets and a C-terminal alpha amphipathic helix. On binding to phosphatidylinositol lipids, a key membrane constituent, PH domains act in the recruiting of proteins to the cell membrane. A large number of PH domains, however, have poor affinity for phosphoinositides and, in fact, function as protein-binding domains. Hence, PH domains are involved in the formation of signalling complexes involved in cell fate decisions and require precise temporal and spatial control. They may employ multiple biologically optimized interaction surfaces that together form a cooperative signalling device.
As for the hierarchy, the best interaction between PAR1&2 C-tails and PH domains takes place via protein–protein interaction, as with Etk/Bmx. However, since these interactions are of high importance, a back-up system is also available in cases when Etk/Bmx is absent in a specific physiological context, for example, in a PH-Akt association.
In summary, PH motifs for binding associations, either with lipids that are located within cellular membranes, or via protein–protein interactions, exemplify how the interplay between distinct motifs in a signal protein not only support transmission of a biochemical signal but also ensure a robust response to developmental cues, at precisely the right time, and with sufficient specificity to safeguard against premature and hence disastrous induction of cell fate change.
Biased signalling at GPCRs has redefined classical concepts in receptor pharmacology, not only highlighting the depth of signalling diversity within the GPCR system but also offering possibilities for more effective therapeutics58,59. We have now explored NE’s role in determining the consequences of PH-Etk/Bmx-binding association with either PAR1 or PAR2. NE did not elicit the association of either of the PH-domain signal proteins analysed, Etk/BMX and Akt, with PAR2 (Supplementary Fig. 10). In contrast, NE was capable of eliciting PAR1 association with both PH-Etk/BMX and PH-Akt (Supplementary Fig. 11). This dichotomy can be explained by the NE cleavage site locations on PAR2 versus PAR1. While the main digestion site of the PAR2 N-terminal extracellular domain is rather far downstream from the canonical trypsin cleavage site and is allocated to residues F(67) and (68)SAS, NE cleaves PAR1 only four residues after the classical thrombin-cleaving site (41)R/(42)SFLLRN, after L residue L(45)/R(46)NPNDKY. Our NE data are still preliminary and were not the focus of the current study. However, it appears that, at least with regard to PAR1, rather than disarming its function, the induced PH-Etk/BMx and/or PH-Akt association joins the MAPK activation previously demonstrated for NE but not for other neutrophil proteinases (for example, proteinase-3 and cathepsin G)60,61,62. The possibility that endogenous NE may be capable of instigating a key PAR1 signalling event, albeit to a diminished extent compared with classical activation, may be important. This proteinase is abundant in the surroundings of a tumour, providing support to the axis in terms of inflammation-induced tumour development.
Other PAR2 C-tail sequences have been described as having an impact on PAR2-induced cellular functions. A sequence region immediately downstream to the PH-domain-binding motif, allocated to residues 356–363, was found to affect PAR2-induced InsP3 accumulation, Ca++ mobilization and PYK activation. However, the role of this sequence is not clear. It was suggested that perhaps it has to do with the deletion of a palmitoylation site that is usually recognized by a cystein flanked by basic amino-acid residues, as is the case with residues 356–363 (ref. 63). In addition, Vogel’s group has demonstrated crosstalk and physical interaction between PAR2 and TLR4, a member of the Toll-like receptor (TLR) family64. TLRs serve as important guards of the innate immune response through their ability to sense conserved pathogen-associated molecular patterns. The C-tail region sequence of PAR2, where the physical interaction with TLR4 takes place, is currently unknown and remains to be fully elucidated. Whether PAR2 PH-domain is critically involved in these interactions is an open question.
The three intracellular loops designated C1, C2 and C3 along with the C-tail, termed C4, are critical for class A GPCR signalling, and are recapitulated by interaction with the heterotrimeric G-proteins (for example, α, β and γ subunits)65. In general, C1 is the shortest in length, with relatively conserved length between family members, whereas the highest degree of variability is found in C3 and C4 (ref. 66). Ligand binding to GPCRs mediates a large conformational change most notably among others, in C2 and C3, which in turn promote activation of G-proteins by exchange of GDP for GTP on the Gα subunit67. An array of components composed of a lipid moiety (for example, palmitate, myristate and lithocholic acid) attached to a peptide that corresponds to an amino-acid segment of the cytoplasmic loops (C1, C2 or C3) or the C-terminal tail have shown however to affect all three intracellular loops and also C4, suggesting that all intracellular domains may be important for signal transduction. Within the C-terminal domain is the highly conserved eighth helix (H8) previously identified in rhodopsin65 and other Class A receptors, including PAR1 (ref. 68) and PAR2 (ref. 69). H8 is anchored to the membrane by palmitoylation of C-terminal cysteine residues. In fact, the PAR1 PH domain is localized within the H8 loop and was found to be confined to this region. Similarly, palmitoylation of PAR2 is necessary for post-translational modification and is required for efficient cell surface expression and desensitization of PAR2. Our data demonstrate for the first time that PH-domain-binding motifs in the PAR1 and PAR2 C-tails are critical signal-initiating sites. These findings define a molecular path in PAR-induced signalling networks. These sites are potential targets for future drug design. It is possible that other cancer ‘driver’ GPCRs harbour PH-domain-binding motifs within their C-tails, which would contribute a more general significance to these sites. This possibility needs to be fully explored.
Methods
Cell culture
HEK-293T, MCF-7, HCT-116 and CL-1 cells (obtained from the American Type Culture Collection) were grown in DMEM. HU breast epithelial cells were generated by the late Dr Aviva Horowitz (member and friend of the Sharett Institute of Oncology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel). The cells were grown in RPMI, supplemented with 1 mM L-glutamine, 50 μg ml−1 streptomycin, 50 U ml−1 penicillin (GIBCO-BRL, Gaithersburg, MD, USA) and 10% fetal calf serum (Biological Industries, Beit Haemek, Israel). Cells were maintained in a humidified incubator with 8% CO2 at 37 °C.
Plasmids and transfection
A cDNA encoding wild-type human Par2 was kindly provided by Professor Morley D. Hollenberg (Faculty of Medicine, University of Calgary, Alberta, Canada). Etk/Bmx viral vector and GST-PH-Etk/Bmx constructs were kindly provided by Dr Yun Qiu (Departments of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD, USA). The GST-PH-Akt construct was kindly provided by Dr Brian A. Hemmings (Friedrich Miescher Institute, Basel, Switzerland). The GST-PH-Vav3 construct was kindly provided by Dr Shan Lu (University of Cincinnati College of Medicine, Cincinnati, OH, USA).
Cells were grown to 70–80% confluency and transfected with 0.1–3.5 μg of plasmid DNA in TransIT LT1 transfection reagent (Mirus Bio LLC, Madison, WI, USA) according to the manufacturer’s instructions. Cells were collected 48 h after transfection and protein lysates/RNA were purified.
MCF-7, HU or HEK 293T were grown to 70–80% confluency and transfected with 1–2 μg of either wt human hPar1 or hPar2 or truncated hPar2 (devoid of the cytoplasmic tail) cDNA, or with several hPar2-deleted constructs, or with a control pcDNA3 vector (Invitrogen, Carlsbad, CA, USA) using TransIT LT1 transfection reagent (Mirus Bio LLC). Transfected cells were selected with G418 (800 μg ml−1) to obtain stable populations of cells expressing hPar1 and hPar2, or hPar1 and truncated hPar2.
PAR1 and PAR2 activation
Thrombin receptor-activating peptides TFLLRNPNDK for activation of PAR1 and SLIGKV for PAR2 were from GenScript (Piscataway, NJ, USA). Thrombin was obtained from OMRIX Bio Pharmaceutical (Ramat Gan, Israel).
Gateway cloning
The wt hPar1 (or) hPar1 7A, wt hPar2 and mutant hPar2 H349A-coding sequence was cloned into a neoR containing the lentiviral vector using the Gateway cloning system according to the manufacturer’s instructions.
Generation and titre of virus
Viral production was performed by co-transfecting neo HA hPar1 vector orneo HA hPar1 7A vector (2 μg) and packaging vectors pCMVΔR8.91 (1.8 μg) and pMD2.VSVG (0.2 μg) into HEK293T cells using 12 μl TransIT LT1 transfection reagent (Mirus Bio LLC) in 100-mm plates. The resulting supernatant was collected after 48 and 72 h. The virus was recovered after ultracentrifugation for 1 h at 42,000 r.p.m. in a Beckman SW28 rotor (Beckman-Coulter, Brea, CA, USA). The resulting pellet was resuspended in medium to reach 100 × the initial viral concentration. Various hPar2 constructs, wt hPar2, and truncated and deleted hPar2 C-tail constructs, as well as mutants of hPar2 were cloned into lentiviral vectors.
Preparation of stable clones
MCF7 cells were infected with HA-hPar1 virus or hPar1-7A virus. This was followed by selection using Geneticin G418 resistance (500 μg ml−1) to produce MCF7-hPar1 and MCF7-hPar1-7A stable clones. To obtain MCF7-hPar1-Etk/Bmx and MCF7-hPar1-7A-Etk/Bmx stable clones, infection of Etk/Bmx virus was performed to the above-mentioned clones. Clone efficiency was evaluated using RT–PCR and western blotting. HU stable clones stably expressing hPar2wt and various deletion constructs, as well as mutants, were similarly generated.
Mammary gland mouse model
Female athymic nude mice aged 6–7 weeks were pre-implanted subcutaneously with pellets containing 1.7 mg β-estradiol (60-day release, Innovative Research of America, Sarasota, FL, USA). Mouse mammary fat pads were then injected with 5 × 106 MCF-7 cells stably expressing hPar1 wt, hPar1 wt and Etk/Bmx, hPar1-7A and hPar1-7A and Etk/Bmx constructs, or pcDNA3 control plasmid. Mice were monitored for tumour size by external calibre measurements (length and width) on days 10, 22, 25 and for up to 45 days if tumour burden allowed. Tumour volume (V) was calculated by V=L × W2 × 0.5, where L is length and W is width. At the end of the experiment, mice were killed and tumours were removed, weighed and fixed in formalin for histology. All animal experiments were approved by the animal committee of the Hebrew University (MD-09-11803-2).
Histology
Tissue samples derived from mouse primary tumours were fixed with 4% formaldehyde in PBS, embedded in paraffin and sectioned (5 μm sections). After deparaffinization and rehydration, the sections were stained with H&E or were subjected to immunohistochemistry using specific antibodies.
Immunohistochemistry
Paraffin-embedded slides were deparaffinized and incubated in 3% H2O2. Antigen unmasking was carried out by heating (20 min) in a microwave oven in 10 mM Tris buffer containing 1 mM EDTA. After blocking, slides were incubated with the following primary antibodies: PCNA (sc-56, Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:200), β-catenin (C-2206, Sigma-Aldrich, St Louis, MO, USA; dilution 1:100) and caspase 3 (9661S, Cell Signaling Technology, Danvers, MA, USA; dilution 1:100) and diluted in CAS-Block (Invitrogen), or with CAS-Block alone, as a control. Appropriate secondary antibodies (Nichirei, Tokyo, Japan; dilution 1:1,000) were then added and the slides were incubated at room temperature for 30 min. Colour was developed using the 3,3′-diaminobenzidine (DAB) (Thermo Scientific, Walham, MA, USA) or the Zymed AEC substrate kit (Zymed Laboratories So., San Francisco, CA, USA), followed by counterstaining with Mayer’s haematoxylin. Controls without addition of primary antibody showed low- or no background staining in all cases.
Generation of PAR2 deletion constructs and mutants
PAR2 deletion mutants were generated with site-directed mutagenesis using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies Stratagene, Santa Clara, CA, USA) according to the manufacturer’s instructions.
The primers used for insertion of stop codons, invariably along the hPar2 C-tail, and for the generation of mutants R352A and H349A, are shown in Supplementary Table 2.
Membrane solubilization
Cells were solubilized in lysis buffer containing 10 mM Tris-HCl (pH 8), 2.5 mM MgCl2, 5 mM KCl, 1 mM dithiothreitol, protease inhibitor mixture (1:100), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM sodium orthovanadate (Sigma-Aldrich) for 30 min at 4 °C. After centrifugation at 12,000g, the supernatant containing the cytoplasmic fraction was collected and the pellet was resuspended with 1% Triton X-100, 150 mM NaCl and 50 mM Tris acetate (pH 8.2). The supernatants obtained after centrifugation at 12,000g contained the membrane fraction. This procedure facilitated enrichment of the membrane fraction.
Western blot analysis and antibodies
Cells were solubilized for 30 min at 4 °C in lysis buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, a protease inhibitor cocktail (0.3 μM aprotinin, 1 mM PMSF; Sigma-Aldrich and 10 μM leupeptin). After centrifugation at 12,000g for 20 min at 4 °C, the supernatants were transferred and the protein content was measured. Lysates (50 μg) were loaded on a 10% SDS–PAGE gel followed by transfer to Immobilon-P membrane (EMD Millipore/Merck, Damstadt, Germany). Membranes were blocked and probed with the appropriate antibodies. Anti-HA for IP (1 μg per assay) was obtained from Santa Cruz Biotechnology, and for Western blot (HA.11mAb; 1:500 dilution) from Covance, Berkeley, USA. 1 μg/assay SAM11 antibody (Santa Cruz Biotechnology) was used for IP and a 1:500 dilution was used for Western blot detection of overexpressed hPar2. Rabbit anti GFP antibody, anti Akt antibody and anti phospho-Akt were obtained from Cell Signaling Technology and used at a dilution of 1:1,000. Mouse anti T7 antibody was obtained from Novagen Madison, WI; and used at a dilution of 1:10,000. Anti β-actin was purchased from Sigma-Aldrich and used at a dilution of 1: 5,000. Uncropped immunbloots and larger blot areas are represented in Supplementary Fig. 12
Immunoprecipitation
Protein cell lysates (400 μg) were used for IP analysis. Mouse anti-PAR2 antibodies were added to the cell lysates. After overnight incubation, protein A-sepharose beads (Sigma-Aldrich) were added to the suspension, which was subsequently rotated at 4°C for 1 h. Elution of the reactive proteins was performed by resuspending the beads in protein sample buffer followed by boiling for 5 min. The supernatant was then resolved on a 10% SDS–polyacrylamide gel and was treated as indicated above for western blot analysis.
GST fusion protein
Fusion proteins were purified from transformed Esherichia coli (strain BL21) that had been stimulated with isopropyl-β-D-thio-galactopyranoside at a concentration of 0.3 μM. Bacteria were lysed by sonication in solution containing 10 mM Tris-HCl (pH 8.0), 0.5% Nonidet P-40 (NP-40), 100 mM sodium chloride, 20 mM EDTA (pH 8.0) and protease inhibitors. Lysates were then immobilized on glutathione sepharose beads (Pharmacia/GE LifeSciences, Marlborough, MA, USA). Lysates of HEK-293 with and without transfection with T7-Etk/Bmx, colon carcinoma CL1, HCT-116 and HU cells were loaded on these columns. The bound proteins were then washed and sample buffer was added and loaded on SDS–PAGE, followed by immunoblotting with the indicated antibodies and ECL detection.
GST-hPAR2-C-tail cloning
The C-tail fragment of hPAR2, containing 52 amino acids from residue 346 (phenylalanine) to residue 397 (tyrosine), was prepared using PCR amplification (using primers containing EcoRI and BamHI restriction sites (respectively, indicated by underlined letters): 5′-CGGAATTCTTTGTTTCACATGATTTCA-3′ and 5′-CGGGATCCATAGGAGGTCTTAACAGT-3′). The resulting DNA fragment was further cut with the appropriate restriction enzymes (BamHI and EcoRI) and ligated into the pGEX-2T vector. GST-hPAR2K378Z-C-tail and GST-hPAR2K356Z-C-tail were also prepared using the same technique. The suitability of the constructs was confirmed by dideoxy sequencing followed by SDS–PAGE separation, which indicated that the fusion proteins of the C-tails were adequately prepared. The molecular weights of GST fusion proteins were as follows: 27 kD for GST itself, 32.9 kD for the GST-hPAR2-C-tail, 30.7 kD for the GST-hPAR2K378Z-C-tail, 28.2 kD for the GST-hPAR2K356Z-C-tail, 45 kD for GST-PH-Akt and 80 kD for GST-PH-Vav3.
GST-PH-Etk/Bmx
The PH domain in Etk/Bmx was bound to the GST column as previously described27. Briefly, GST fusion proteins and His-T7-tagged proteins were expressed in bacteria and purified by using glutathione sepharose (Pharmacia) or Ni2+ column (Novagen) as recommended by the manufacturers. The purified GST fusion proteins remained on the glutathione sepharose beads and were then mixed with purified His-T7-tagged PH domain in PBS containing 0.5 mg ml−1 bovine serum albumin. After overnight incubation, the beads were collected and extensively washed with cold PBS. The bound proteins were analysed using SDS–PAGE followed by western blot analysis with anti-T7 antibody. The GST-PH-Akt construct was kindly provided by Dr Brian A. Hemmings (Friedrich Miescher Institute). The GST-PH-Vav3 construct was kindly provided by Dr Shan Lu (University of Cincinnati College of Medicine).
HA-tag wt hPar1 and HA-mutant hPar1-7A C-tail constructs
Mutants were designed for insertion of A at the carboxy terminus of PAR1 residues 378–384: SSECQRYVYSILCCKESS to SSEAAAAAAAILCC (named hPar1-7A mutant). For HA-tag wt hPar1 construct PCR primers were designed and added downstream to the ATG start codon. Primers for the HA-tag were as follows: sense: 5′-TACCCATACGATGTTCCAGATTACGCT-3′ and antisense: 5′-AGCGTAATCTGGAACATCTATGGGTA-3′. Replacement of seven residues with Ala (A) at positions 378–384 was performed by synthesis of oligos containing the mutation. Primer sequences were as follows: hPar1 7A mutant: sense: 5′-TCTGAGGCTGCTGCTGCTGCTGCAGCTATCTTA-3′ and antisense: 5′-TAAGATAGCTGCAGCAGCAGCAGCAGCCTCAGA-3′. PCR products were then used as primers on an hPar1 cDNA template to create an extended product of mutations introduced into the full-length sequence. The amplified DNA fragment was digested with PinAI and XbaI from PAR1 cDNA and was cloned into pcDNA3-hPar1 plasmid followed by DNA sequencing.
Placenta tissue collection and preparation
Placental tissues were prepared as previously described35. Briefly, the placental tissues were extracted from discarded material provided by patients who voluntarily and legally chose to terminate pregnancy during the first trimester (between 5- and 12-week gestation). Gestational age was determined by the date of the last menstrual period and ultrasound measurement of fetal crown-rump length. All specimens were obtained strictly in adherence with the Hadassah-Hebrew University Institutional Ethics Committee Guidelines (we received informed consent from women who voluntarily and legally chose to terminate pregnancy; Helsinky 0436-13-HMO; serial number 123130).
First-trimester villous explants in cultures
The preparation and cultivation of villous explants of various first-trimester placentae was performed as described elsewhere20,35,36,37. Briefly, placental tissue originating from samples harvested between 10- and 12-week gestation was immediately rinsed in sterile cold PBS and processed within 2 h of collection. Following dissection under the microscope, ∼2–5 mg weight specimens were carefully laid on 200 μl of solid reconstituted undiluted Matrigel substrate (Becton Dickinson, Bedford, MA, USA) in 0.4-μm pore-culture Millicell-CM culture dish inserts (pore size 0.4 μm, Millipore Corp, Bedford, MA, USA). Explants were cultured in DMEM/F-12 supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 0.25 μg ml−1 ascorbic acid, pH 7.4. Villous explants were maintained in culture for 3 days. Viability of the explants was assessed by adherence to Matrigel and emerging EVTs breaking from the tips as observed under a phase microscope. After 24 h in culture, explants were treated with thrombin (1 U ml−1), TFLLRN (100 μM) and SLIGKV (100 μM), and were transfected or not with wt hPar1, hPar1 7A mutant or with wt hPar2 and mutant hPar2 H349A viral vectors. After 72 h in culture, the Matrigel cast cylinders with the EVTs were detached from the base of the insert cells by fine dissection under the microscope, fixed in 4% paraformaldehyde, embedded in paraffin and further processed for histochemical tissue analysis. Each experiment was carried out in triplicate using samples originating from eight different placental sets.
EVT invasion assessment
We assessed the H&E-stained serial sections of the EVT. We limited our study to the trophoblasts that penetrated through the Matrigel but remained on the surface of the polycarbonate membrane, that is, within the cast35,36,37. These were visualized on sequential slices of the entire Matrigel cast by haematoxylin staining. Slides were photographed using the digital image capture software and all specimens were evaluated by two authors (S.G.-G. and M.M.). Depth of invasion was assessed for each experiment (disappearance of trophoblast cells on the serial sections). For each set of experimental conditions, the total number of EVT cells was counted and the mean±s.e.m./high power field (HPF) was calculated for five randomly selected microscope fields (magnification × 40) at the deepest common level of invasion.
Statistical analysis
At the deepest common level of invasion, the total EVT cells at five randomly selected microscope fields were counted, for each experimental condition and the mean±s.e.m./HPF calculated. The data were statistically evaluated using analysis of variance Tukey honest significant difference (HSD) test of multiple comparison showing a P value of 0.0001 within groups. The mean difference is significant at the 0.05 level.
Additional information
How to cite this article: Kancharla, A. et al. PH motifs in PAR1&2 endow breast cancer growth. Nat. Commun. 6:8853 doi: 10.1038/ncomms9853 (2015).
References
Dorsam, R. T. & Gutkind, J. S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94 (2007).
Lappano, R. & Maggiolini, M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 10, 47–60 (2011).
Feigin, M. E. Harnessing the genome for characterization of G-protein coupled receptors in cancer pathogenesis. FEBS J. 280, 4729–4738 (2013).
Santos, R. A. et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl Acad. Sci. USA 100, 8258–8263 (2003).
Debnath, J., Walker, S. J. & Brugge, J. S. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J. Cell Biol. 163, 315–326 (2003).
Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904 (2006).
Lopez-Otin, C. & Hunter, T. The regulatory crosstalk between kinases and proteases in cancer. Nat. Rev. Cancer 10, 278–292 (2010).
Coughlin, S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000).
Rasmussen, U. B. et al. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 288, 123–128 (1991).
Nystedt, S., Emilsson, K., Wahlestedt, C. & Sundelin, J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl Acad. Sci. USA 91, 9208–9212 (1994).
Adams, M. N. et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol. Ther. 130, 248–282 (2011).
Soh, U. J., Dores, M. R., Chen, B. & Trejo, J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 160, 191–203 (2010).
Bar-Shavit, R. et al. PAR1 plays a role in epithelial malignancies: transcriptional regulation and novel signaling pathway. IUBMB Life 63, 397–402 (2011).
Booden, M. A., Eckert, L. B., Der, C. J. & Trejo, J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol. Cell Biol. 24, 1990–1999 (2004).
Even-Ram, S. et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat. Med. 4, 909–914 (1998).
Versteeg, H. H. et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res. 68, 7219–7227 (2008).
Blackhart, B. D. et al. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 271, 16466–16471 (1996).
O'Brien, P. J. et al. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J. Biol. Chem. 275, 13502–13509 (2000).
Sevigny, L. M. et al. Protease-activated receptor-2 modulates protease-activated receptor-1-driven neointimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 31, e100–e106 (2011).
Jaber, M. et al. Protease-activated-receptor-2 affects protease-activated-receptor-1-driven breast cancer. Cell Mol. Life Sci. 71, 2517–2533 (2014).
Lemmon, M. A. & Ferguson, K. M. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350, (Pt 1): 1–18 (2000).
Rebecchi, M. J. & Scarlata, S. Pleckstrin homology domains: a common fold with diverse functions. Annu. Rev. Biophys. Biomol. Struct. 27, 503–528 (1998).
Song, G., Ouyang, G. & Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol. Med. 9, 59–71 (2005).
Hanada, M., Feng, J. & Hemmings, B. A. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim. Biophys. Acta 1697, 3–16 (2004).
Neet, K. & Hunter, T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cells 1, 147–169 (1996).
Smith, C. I. et al. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays 23, 436–446 (2001).
Cohen, I. et al. Etk/Bmx regulates proteinase-activated-receptor1 (PAR1) in breast cancer invasion: signaling partners, hierarchy and physiological significance. PLoS ONE 5, e11135 (2010).
Zeng, L. et al. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Cell Biol. 20, 9212–9224 (2000).
Lee, K. et al. Vav3 oncogene activates estrogen receptor and its overexpression may be involved in human breast cancer. BMC Cancer 8, 158 (2008).
Rao, S. et al. A novel nuclear role for the Vav3 nucleotide exchange factor in androgen receptor coactivation in prostate cancer. Oncogene 31, 716–727 (2012).
Cote, J. F., Motoyama, A. B., Bush, J. A. & Vuori, K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat. Cell Biol. 7, 797–807 (2005).
McManus, E. J. et al. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23, 2071–2082 (2004).
Cenni, B., Gutmann, S. & Gottar-Guillier, M. BMX and its role in inflammation, cardiovascular disease, and cancer. Int. Rev. Immunol. 31, 166–173 (2012).
Gottar-Guillier, M. et al. The tyrosine kinase BMX is an essential mediator of inflammatory arthritis in a kinase-independent manner. J. Immunol. 186, 6014–6023 (2011).
Bischof, P. & Irminger-Finger, I. The human cytotrophoblastic cell, a mononuclear chameleon. Int. J. Biochem. Cell Biol. 37, 1–16 (2005).
Genbacev, O., Schubach, S. A. & Miller, R. K. Villous culture of first trimester human placenta--model to study extravillous trophoblast (EVT) differentiation. Placenta 13, 439–461 (1992).
Grisaru-Granovsky, S. et al. Protease activated receptor-1, PAR1, promotes placenta trophoblast invasion and beta-catenin stabilization. J. Cell Physiol. 218, 512–521 (2009).
Konishi, H., Kuroda, S. & Kikkawa, U. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase C zeta. Biochem. Biophys. Res. Commun. 205, 1770–1775 (1994).
Yao, L., Kawakami, Y. & Kawakami, T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc. Natl Acad. Sci. USA 91, 9175–9179 (1994).
Farhang-Fallah, J. et al. The pleckstrin homology (PH) domain-interacting protein couples the insulin receptor substrate 1 PH domain to insulin signaling pathways leading to mitogenesis and GLUT4 translocation. Mol. Cell Biol. 22, 7325–7336 (2002).
Myers, M. G. Jr. et al. The pleckstrin homology domain in insulin receptor substrate-1 sensitizes insulin signaling. J. Biol. Chem. 270, 11715–11718 (1995).
Pitcher, J. A., Touhara, K., Payne, E. S. & Lefkowitz, R. J. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J. Biol. Chem. 270, 11707–11710 (1995).
Scheffzek, K. & Welti, S. Pleckstrin homology (PH) like domains - versatile modules in protein-protein interaction platforms. FEBS Lett. 586, 2662–2673 (2012).
Zheng, Y., Zangrilli, D., Cerione, R. A. & Eva, A. The pleckstrin homology domain mediates transformation by oncogenic dbl through specific intracellular targeting. J. Biol. Chem. 271, 19017–19020 (1996).
Chen, Z. et al. Activated RhoA binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. J. Biol. Chem. 285, 21070–21081 (2010).
Cohen, L. A. et al. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol. Biol. Cell 18, 2244–2253 (2007).
Lutz, S. et al. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science 318, 1923–1927 (2007).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Stocker, H. et al. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295, 2088–2091 (2002).
Franke, T. F. et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 (1995).
Carpten, J. D. et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448, 439–444 (2007).
Jia, Y. et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity 27, 453–467 (2007).
Jia, Y., Schurmans, S. & Luo, H. R. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle 7, 2803–2808 (2008).
Rawlings, D. J. et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261, 358–361 (1993).
Chen, R. et al. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat. Cell Biol. 3, 439–444 (2001).
Movilla, N. & Bustelo, X. R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell Biol. 19, 7870–7885 (1999).
Bustelo, X. R. The VAV family of signal transduction molecules. Crit. Rev. Oncog. 7, 65–88 (1996).
Hollenberg, M. D. et al. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br. J. Pharmacol. 171, 1180–1194 (2014).
Zhao, P., Metcalf, M. & Bunnett, N. W. Biased signaling of protease-activated receptors. Front. Endocrinol. (Lausanne) 5, 67, 1-16 (2014).
Mihara, K., Ramachandran, R., Renaux, B., Saifeddine, M. & Hollenberg, M. D. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J. Biol. Chem. 288, 32979–32990 (2013).
Ramachandran, R. et al. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J. Biol. Chem. 286, 24638–24648 (2011).
Zhao, P. et al. Neutrophil elastase activates protease-activated receptor-2 (PAR2) and transient receptor potential vanilloid 4 (TRPV4) to cause inflammation and pain. J. Biol. Chem. 290, 13875–13887 (2015).
Seatter, M. J. et al. The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell Signal. 16, 21–29 (2004).
Rallabhandi, P. et al. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J. Biol. Chem. 283, 24314–24325 (2008).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000).
Mirzadegan, T., Benko, G., Filipek, S. & Palczewski, K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry 42, 2759–2767 (2003).
Gether, U. & Kobilka, B. K. G protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 273, 17979–17982 (1998).
Swift, S. et al. Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J. Biol. Chem. 281, 4109–4116 (2006).
Adams, M. N., Christensen, M. E., He, Y., Waterhouse, N. J. & Hooper, J. D. The role of palmitoylation in signalling, cellular trafficking and plasma membrane localization of protease-activated receptor-2. PLoS ONE 6, e28018 (2011).
Acknowledgements
We are grateful to Shifra Fraifeld for English editing of the manuscript. This work is supported by grants of the Israel Academy of Sciences and Humanities and Nofar Chief Israeli Scientist, Trade and Industrial Office to B-SR.
Author information
Authors and Affiliations
Contributions
M.M., K.A., J.M. and A.D. performed experiments; M.M., G.-G.S., U.B. and P.T. designed research and analysed data; B.-S.R., M.M. and G.-G. S. designed research, analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-12 and Supplementary Tables 1-2 (PDF 1635 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kancharla, A., Maoz, M., Jaber, M. et al. PH motifs in PAR1&2 endow breast cancer growth. Nat Commun 6, 8853 (2015). https://doi.org/10.1038/ncomms9853
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9853
This article is cited by
-
Cancer driver G-protein coupled receptor (GPCR) induced β-catenin nuclear localization: the transcriptional junction
Cancer and Metastasis Reviews (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.