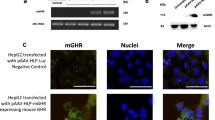
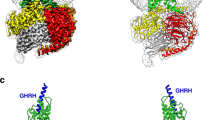
Abstract
The growth hormone–releasing hormone receptor (GHRHR) is a member of the family of G protein–coupled receptors that is expressed on pituitary somatotrope cells and mediates the actions of GHRH in stimulating growth hormone (GH) synthesis and secretion. We report that the Ghrhr gene is located in the middle of mouse chromosome 6 in the same region as the little mutation. Mice homozygous for this mutation have reduced GH secretion and a dwarf phenotype. A missense mutation was identified in the extracellular domain of the little GHRHR that disrupts receptor function, suggesting that the growth deficit in these mice results from a defect in the GHRHR. Similar alterations in GHRHR might explain some isolated GH deficiencies in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Eicher, E.M. & Beamer, W.G. Inherited ateliotic dwarfism in mice: characterization of the mutation, little, on chromosome 6. J. Hered. 67, 87–91 (1976).
Beamer, W.G. & Eicher, E.M. Stimulation of growth in the little mouse. J. Endocrinol. 71, 37–45 (1976).
Hammer, R.E., Palmiter, R.D. & Brinster, R.L. Partial correction of murine hereditary growth disorder by germ-line incorporation of a new gene. Nature 311, 65–67 (1984).
Jansson, J.-O., Downs, T.R., Beamer, W.G. & Frohman, L.A. Receptor-associated resistance to growth hormone-releasing factor in dwarf “little” mice. Science 232, 511–512 (1986).
Guillemin, R. et al. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science 218, 585–587 (1982).
Rivier, J., Spiess, J., Thorner, M. & Vale, W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature 300, 276–278 (1982).
Mayo, K.E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Molec. Endocrinol. 6, 1734–1744 (1992).
Gaylinn, B.D. et al. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone. Molec. Endocrinol. 7, 77–84 (1993).
Lin, C., Lin, S.-C., Chang, C.-P. & Rosenfeld, M.G. Pit–1 dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature 360, 765–768 (1992).
Gonzalez, G.A. & Montminy, M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–689
Sheng, M., Thompson, M.A. & Greenberg, M.E. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252, 1427–1430
Bodner, M. et al. The pituitary-specific transcription factor GHF–1 is a homeobox-containing protein. Cell 55, 505–518 (1988).
Ingraham, H. et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell 55, 519–529 (1988).
McCormick, A., Brady, H., Theill, L.E. & Karin, M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmentalcues. Nature 345, 829–832
Struthers, R.S., Vale, W.W., Arias, C., Sawchenko, P.E. & Montminy, M.R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature 350, 622–624
Copeland, N.G. & Jenkins, N.A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 7, 113–118 (1991).
Donahue, L.R. & Beamer, W.G. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 (IGFBP–3), but does not affect IGFBP−2, −1 or −4. J. Endocrinol. 136, 91–104 (1993).
Mayo, K.E. et al. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Molec. Endocrinol. 2, 606–612 (1988).
Cheng, T.C. et al. Etiology of growth hormone deficiency in little, Ames and Snell dwarf mice. Endocrinol. 133, 1669–1678 (1983).
Ishahara, T. et al. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO Journal 10, 1635–1641 (1991).
Ishihara, T., Shigemoto, R., Mori, K., Takahashi, K. & Nagata, S. Functional expression and tissue distribution of anovel receptor for vasoactive intestinal polypeptide. Neuron 8, 811–819 (1992).
Jelinek, L.J. et al. Expression cloning and signaling properties of the rat glucagon receptor. Science 259, 1614–1616 (1993).
Thorens, B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. natn. Acad. Sci. U.S.A. 89, 8641–8645 (1992).
Juppner, H. et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254, 1024–1026 (1991).
Lin, H. et al. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science 254, 1022–1024 (1991).
Snell, G.D. “Dwarf” a new Mendelian recessive character of the house mouse. Proc. natn. Acad. Sci. U.S.A. 15, 733–734 (1929).
Li, S. et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347, 528–533 (1990).
Schaible, R. & Gowen, J.W. A new dwarf mouse. Genetics 46, 896 (1961).
Wilson, D.B., Wyatt, D.P., Gadler, R.M. & Baker, C.A. Quantitative aspects of growth hormone cell maturation in the normal and little mutant mouse. Acta Anat. 131, 150–155 (1988).
Asa, S.L. et al. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinol. 131, 2083–2089 (1992).
Thorner, M.O. et al. Somatotroph hyperplasia: successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J. clin. Invest. 70, 965–977 (1982).
Sano, T., Asa, S.L. & Kovacs, K. Growth hormone-releasing hormone producing tumors: clinical, biochemical and morphological manifestations. Endocrine Rev. 9, 357–373 (1988).
Horvath, S., Palkovits, M., Gorecs, T. & Amimura, A. Electron microscopic immunocytochemical evidence for the existence of bidirectional synaptic connections between growth hormone releasing hormone and sommatostatin neurons in the hypothalamus. Brain Res. 481, 8–15 (1989).
Phelps, C.J. & Hoffman, G.E. Isolated deficiency of immunocytochemically detected somatostatin in Snell dwarf, but not in “little”, mice. Peptides 8, 1127–1133 (1987).
Suhr, S.T., Rahal, J.O. & Mayo, K.E. Mouse growth hormone-releasing hormone: precursor structure and expression in brain and placenta. Molec. Endocrinol. 3, 1693–1700 (1989).
Margioris, A.N. et al. Expression and localization of growth hormone-releasing hormone messenger ribonucleic acid in the rat placenta: in vitro secretion and regulation of its peptide product. Endocrinol. 126, 151–158 (1990).
Meigan, G., Sasaki, A. & Yoshinaga, K. Immunoreactive growth hormone-releasing hormone in rat placenta. Endocrinol. 123, 1098–1102 (1988).
Spatola, E. et al. Interaction of growth hormone-releasing hormone with the insulin-like growth factors during prenatal development in the rat. Endocrinol. 129, 1193–1200 (1991).
Bagnato, A., Moretti, C., Ohnishi, J., Frajese, G. & Catt, K. Expression of the growth hormone-releasing hormone gene and its peptide product in the rat ovary. Endocrinol. 130, 1097–1102 (1992).
Berry, S.A. & Pescovitz, O.H. Ontogeny and pituitary regulation of testicular growth hormone-releasing hormone-like messenger RNA. Endocrinol. 127, 1404–1411 (1990).
Moretti, C., Bagnato, A., Solan, N., Frajese, G. & Catt, K. Receptor-mediated actions of growth hormone releasing factor on granulosa cell differentiation. Endocrinol. 127, 2117–2126 (1990).
Chiampani, T., Fabbri, A., Isidori, A. & Dufau, M.L. Growth hormone-releasing hormone is produced by rat Leydig cell in culture and acts as a positive regulator of Leydig cell function. Endocrinol. 131, 2785–2792 (1992).
Dryja, T.P., Hahn, L.B., Cowley, G.S., McGee, T.L. & Berson, E.L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc. natn. Acad. Sci. U.S.A. 88, 9370–9374 (1991).
Rosenfeld, P.S. et al. A Null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nature Genet. 1, 209–213 (1992).
Rosenthal, W. et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature 359, 233–235 (1992).
Pan, Y., Metzenberg, A., Das, S., Jing, B. & Gitschier, J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nature Genet. 2, 103–106 (1992).
Robbins, L.S. et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72, 827–834 (1993).
Underwood, L.E. & Van Wyk, J.J. Normal and aberrant growth. In William's Textbook of Endocrinology (eds Wilson, J.D. & Foster, D.W.) 155–205 (Saunders, Philadelphia, 1985).
MacGillivray, M.H. Disorders of growth and development. In Endocrinology and Metabolism (eds Felig, P., Baxter, J.D., Broadus, A.E. & Frohman, L.A.) 1581–1628 (McGraw-Hill, New York, 1987).
Jenkins, N.A., Copeland, N.G., Taylor, B.A. & Lee, B.K., Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J. Virol. 43, 26–36 (1982).
Siracusa, L.D. et al. Chromosomal location of the octamer transcription factors Otf–1, Otf–2 and Otf–3, defines multiple Otf–3-related sequences dispersed in the mouse genome. Genomics 10, 313–326 (1991).
Green, E.L. Linkage, recombination and mapping. In Genetics and Probability in Animal Breeding Experiments. 77–113 (Oxford University Press, New York, 1981).
Harpold, M.M., Evans, R.M., Salditt-Georgieff, M. & Darnell, J.E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell 17, 1025–1035 (1979).
Heller, D.L., Gianola, K.M. & Leinwand, L.A. A highly conserved mouse gene with a propensity to form pseudogenes in mammals. Molec. Cell Biol. 8, 2797–2803 (1989).
Camp, T.A., Rahal, J.O. & Mayo, K.E. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Molec. Endocrinol. 5, 1405–1417 (1991).
Arrufo, A. & Seed, B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc. natn. Acad. Sci. U.S.A. 84, 8573–8577 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Godfrey, P., Rahal, J., Beamer, W. et al. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet 4, 227–232 (1993). https://doi.org/10.1038/ng0793-227
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0793-227
This article is cited by
-
Pituitary stem cells: past, present and future perspectives
Nature Reviews Endocrinology (2024)
-
Growth hormone releasing hormone signaling promotes Th17 cell differentiation and autoimmune inflammation
Nature Communications (2023)
-
Mice with gene alterations in the GH and IGF family
Pituitary (2022)
-
Mouse models of growth hormone deficiency
Reviews in Endocrine and Metabolic Disorders (2021)
-
Genetical genomics of growth in a chicken model
BMC Genomics (2018)