Abstract
Common mitochondrial DNA (mtDNA) haplotypes in humans and mice have been associated with various phenotypes, including learning performance and disease penetrance. Notably, no influence of mtDNA haplotype in cell respiration has been demonstrated. Here, using cell lines carrying four different common mouse mtDNA haplotypes in an identical nuclear background, we show that the similar level of respiration among the cell lines is only apparent and is a consequence of compensatory mechanisms triggered by different production of reactive oxygen species. We observe that the respiration capacity per molecule of mtDNA in cells with the NIH3T3 or NZB mtDNA is lower than in those with the C57BL/6J, CBA/J or BALB/cJ mtDNA. In addition, we have determined the genetic element underlying these differences. Our data provide insight into the molecular basis of the complex phenotypes associated with common mtDNA variants and anticipate a relevant contribution of mtDNA single nucleotide polymorphisms to phenotypic variability in humans.
Similar content being viewed by others
Main
The mitochondrial oxidative phosphorylation (OXPHOS) system consists of five complexes, four of which are assembled with proteins encoded in the nuclear and the mitochondrial genomes. mtDNA shows extensive sequence variability in mammals, which has been considered to be a consequence of neutral base substitutions accumulated throughout female history in discrete geographical environments owing to genetic drift. Some findings, however, have challenged this view. Thus, common human mtDNA variants may influence sperm motility1,2, longevity3,4,5 or penetrance of some mtDNA-linked diseases6,7, or may predispose to various complex disorders8,9. In addition, adaptive selection may have shaped mtDNA evolution in humans10. In mice, a polymorphism in the tRNA arginine, mitochondrial gene (mt-Tr) modulates the deafness phenotype promoted by the age-related hearing loss (AHL) mutant in Cdh23 (refs. 11,12). Experiments generating mouse embryos with two variants of mtDNA (NZB/B1NJ and BALB/cJ) have shown that liver and kidney systematically accumulate NZB/B1NJ mtDNA, whereas blood and spleen preferentially select BALB/cJ mtDNA13. In addition, mice with different mtDNA (CBA or NZB/B1NJ) substantially differ in their learning capabilities, their sensory development and brain anatomy, owing, to a significant extent, to the mtDNA haplotype14.
Although all mtDNA-encoded genes are involved in biogenesis of the OXPHOS system, studies done in cultured primary cells with different human or mouse mtDNA haplotypes concluded that the respiratory capacity is not substantially influenced by any of the mtDNA variants tested15,16. This apparent contradiction has raised concerns about the significance of the above-mentioned associations9,17.
Here we have analyzed the oxidative phosphorylation capacity of engineered mouse cell lines carrying a homogeneous nucleus but mtDNA derived from C57BL/6J, BALB/cJ, CBA/J or NZB/B1NJ mice or from NIH3T3 mouse cells, as representatives of the mtDNA variants that modulate AHL. Our results show that mtDNA haplotypes from NIH3T3 and NZB/B1NJ mice induce significantly different OXPHOS performance as compared with those from C57BL/6J, BALB/cJ and CBA/J mice. In addition, we conclude that a single nucleotide polymorphism (SNP) in the mt-Tr gene is the sequence variation responsible for the phenotypes observed. The differences in OXPHOS performance are masked by a specific upregulation in mitochondrial biogenesis, triggered by an increase in generation of reactive oxygen species (ROS), in cells carrying NIH3T3 or NZB/B1NJ mtDNA. We show that common and 'non-pathological' mtDNA variants in mice determine differences in OXPHOS performance and ROS production, providing a biochemical and molecular explanation for the above-mentioned associations.
Results
Generation of mouse cybrids with different mtDNA haplotypes
We analyzed seven cell lines generated by transferring mitochondria from the desired source to a mtDNA-less receptor cell line18, ρ0L929neo (Methods). The resulting cell lines ('transmitochondrial cybrids'), which were homogeneous for their nuclear genome but differed in their mtDNA haplotype, were TmC57BL/6J (carrying the mtDNA of C57BL/6J), TmNZB/B1NJ, TmCBA/J, TmBALB/cJ and three independent clones carrying the NIH3T3 mtDNA (TmNIH3T3). In addition, for control purposes, we also included a transmitochondrial cybrid cell line (TmND6ko), generated in our laboratory, carrying the mtDNA of NIH3T3 and the 13887delC mutation in the mt-Nd6 gene. This mutation interferes with the expression of ND6 and the assembly of complex I (refs. 18–20). We carried out restriction fragment length polymorphism analysis (Supplementary Fig. 1 and Supplementary Methods online) to exclude the presence of any trace of the mtDNA from the L929 parental cell line.
We sequenced the mtDNA from CBA/J and NIH3T3 and reanalyzed the full sequence of the mtDNA from NZB/B1NJ to correct for potential errors in the published NZB/B1NJ sequence21. To identify nucleotide differences, these three sequences and those from C57BL/6J and BALB/cJ (ref. 22) were compared by using C57BL/6J as the mtDNA reference sequence (Table 1). We found that CBA/J and BALB/cJ mtDNAs are identical and differ from the reference sequence in only three positions: a synonymous polymorphism in the mt-Nd3 gene, a valine to alanine missense substitution in mt-Co3, and an insertion of an adenine at nucleotide position 9821 in the mt-Tr gene that enlarges the DHU loop of the tRNAArg in one base (Table 1). NIH3T3 mtDNA differs from the C57BL/6J sequence in two positions: the synonymous polymorphism in the mt-Nd3 gene and the insertion in the DHU loop of the tRNAArg. These positions also differ between CBA/J or BALB/cJ and C57BL/6J; however, in this case two adenines are inserted in the NIH3T3 mtDNA sequence, making the DHU loop of the tRNAArg even larger (Table 1).
The mtDNA sequence of NZB/B1NJ is very divergent from that of C57BL/6J (Supplementary Table 1 online). Of note, NZB/B1NJ and NIH3T3 mtDNAs are identical at the three positions where CBA/J and BALB/cJ mtDNAs show differences to the reference sequence. Therefore, among the mtDNA sequences included in our set of transmitochondrial cybrids, there are three different tRNAArg variants with tracks of eight adenines (TmC57BL/6J), nine adenines (TmCBA/J and TmBALB/cJ) and ten adenines (TmNZB/B1NJ and TmNIH3T3) in the DHU loop (Table 1). Because the mtDNA sequences of TmCBA/J and TmBALB/cJ are identical, we considered both cell lines as representative of the same mtDNA haplotype (TmCBA-BALB/cJ).
mtDNA haplotypes promote different growth in galactose
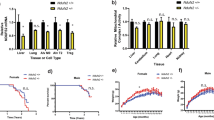
A previous analysis of the respiration capabilities of primary hepatocytes carrying mostly NZB/B1NJ or BALB/cJ mtDNA found no differences16. We readdressed this issue and found no differences in coupled oxygen consumption rates (Fig. 1a, left) or uncoupled respiration (data not shown) among the intact transmitochondrial cybrid lines with the different mtDNA haplotypes. Concordant with this, polarographic measurements of respiratory chain complexes done in digitonin-permeabilized cells showed no differences between the transmitochondrial cybrids except for TmND6ko, the complex I–knockout cell line (Fig. 1b). No significant differences were observed in the extent of coupling between electron transport activity and ATP production, estimated as the ADP/O ratio, either with glutamate plus malate (2.7 ± 0.2, 2.2 ± 0.5, 2.5 ± 0.3 and 2.2 ± 0.6 for TmC57BL/6J, TmCBA-BALB/cJ, TmNIH3T3 and TmNZB/B1NJ, respectively; mean ± s.d.) or with succinate (1.7 ± 0.2, 1.5 ± 0.3, 1.5 ± 0.4 and 1.7 ± 0.1 for TmC57BL/6J, TmCBA-BALB/cJ, TmNIH3T3 and TmNZB/B1NJ, respectively).
(a) Oxygen consumption rate of intact cells with different mtDNA haplotypes (eight to ten adenines at the mt-Tr gene) without any drug (left) or in the presence of NAC (middle) or Tiron (right). (b) Oxygen consumption of permeabilized cells with different mtDNA haplotypes in the presence of electron donors for complex I (glutamate plus malate), complex III (succinate plus glycerol-3-phosphate (G3P)) or complex IV (N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD)). (c) Growth ratio (doubling time ratio) for each cell haplotype in medium containing galactose versus medium containing glucose (Methods), and without any drug (left) or in the presence of NAC (middle) or Tiron (right). Asterisks in a,b indicate a significant difference versus TmC57BL/6J cells (see Supplementary Note online). Asterisks in c indicate that TmND6ko cells do not grow in galactose and that TmND6ko, TmNIH3T3 and TmNZB/B1NJ cells do not grow in galactose in the presence of Tiron. Data are mean ± s.d.
A second parameter that establishes the tightness of the coupling between respiration and ATP synthesis is the respiratory control ratio (RCR), estimated as the ratio between the mitochondrial respiration rate in the presence and that in the absence of ADP (synthesizing versus non-synthesizing ATP). As expected, the RCR for TmC57BL/6J and TmCBA-BALB/c cells was higher with glutamate plus malate, substrates that allow the generation of NADH to deliver electrons to complex I, than with succinate, a substrate for complex II (3.0 ± 0.2 versus 2.1 ± 0.5 for TmC57BL/6J, analysis of variance (ANOVA), P < 0.023; Mann-Whitney U-test, P = 0.020; and 2.6 ± 0.6 versus 2.1 ± 0.4 for TmCBA-BALB/cJ, ANOVA P < 0.034, Mann-Whitney U-test, P = 0.016). However, RCR values were similar with both substrates for TmNIH3T3 and TmNZB/B1NJ cells (2.3 ± 0.3 versus 2.3 ± 0.4 for TmNIH3T3; and 2.3 ± 0.4 versus 2.1 ± 0.6 for TmNZB/B1NJ), owing to the decrease in RCR with glutamate plus malate. No significant differences in the relative amounts of respiratory complexes, estimated by western blotting, were found among the various mtDNA haplotypes (Supplementary Fig. 2 and Supplementary Methods online).
We also observed significant differences (ANOVA, P < 0.0001; Kruskal-Wallis test, P = 0.0061) in the relative ability of the different cell lines to grow in galactose versus glucose (Fig. 1c, left). Thus, whereas cells with the C57BL/6J, BALB/cJ or CBA/J mtDNA showed a similar doubling-time with both substrates (growth ratio 1.01, 0.98 or 0.97, respectively), cells with NIH3T3 or NZB/B1NJ mtDNA showed a delayed growth in galactose with respect to glucose (growth ratio 1.20 or 1.12, respectively; ANOVA Fisher's protected least significant difference (PLSD) post-hoc test for any of the ten-adenine cell lines versus any of the nine- or eight-adenine cell lines, P < 0.05). TmND6ko cells did not grow in galactose.
mtDNA haplotypes promote differential production of H2O2
In addition to ATP and heat production, the mitochondrial respiratory chain is responsible for generating ROS, which can have toxic effects23 but may also have a regulatory role in the control of cell function and particularly in mitochondrial biogenesis24,25. The analysis of ROS production showed that H2O2 levels in TmC57BL/6J and TmCBA-BALB/c cells were similar, but significantly lower than those in TmNZB/B1NJ and TmNIH3T3 cells (Fig. 2a, left). Of note, the TmND6ko cells lacking complex I had the largest quantities of H2O2. To confirm the physiological relevance of the measured ROS concentrations, we cultured all cells in the presence of either N-acetyl-L-cysteine (NAC), a widely used antioxidant and a precursor of reduced glutathione that functions as an H2O2 scavenger, or 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron), a superoxide anion scavenger. One week of culture in the presence of NAC markedly reduced the H2O2 measured in every cell line to similar amounts (Fig. 2a, middle). Three days of treatment with Tiron also abolished the differences in H2O2 among the cell lines (Fig. 2a, right).
(a) Relative amount of H2O2 in cells with different mtDNA haplotypes without drugs (left) or in the presence of NAC (middle) or Tiron (right). The amount of H2O2 in cell line TmC57BL/6J was set to 100. (b) Catalase-specific activity of cells with different mtDNA haplotypes. (c) The mtDNA haplotype determines the mtDNA/nuclear DNA ratio, which in turn is differentially affected by NAC and Tiron. Shown is the mtDNA/nDNA ratio in transmitochondrial cybrids with different mtDNA haplotypes without drug (left) or in the presence of NAC (middle) or Tiron (right). Asterisks indicate a significant difference versus TmC57BL/6J cells (see Supplementary Note). Data are mean ± s.d.
Because ROS generation induces an increase in natural ROS defenses26, we investigated whether the expression of ROS defense enzymes also differed. Catalase activity was lower in cells with C57BL/6J mtDNA, higher in cells with BALB/cJ or CBA/J mtDNA and higher again in cells with the NIH3T3 or NZB/B1NJ mtDNA. TmND6ko cells showed the highest relative catalase activity (Fig. 2b). This observation, together with the evaluation of ROS described above, indicates that the cell lines performing worse in galactose (TmNIH3T3 and TmNZB/B1NJ) have higher ROS production as compared with the other transmitochondrial cybrids. We stress that, because the natural ROS defenses are also modulated in response to ROS, the observed changes in H2O2 may be an underestimation of the true differences in ROS production induced by the mtDNA haplotype.
Cells that produce more ROS have higher mtDNA content
It has been proposed that alterations in intracellular ROS concentrations induce changes in mitochondrial mass and mtDNA copy number25. We therefore analyzed the mtDNA content of the different cell lines by quantitative real-time PCR (Fig. 2c, left). We found that the cells producing more ROS (TmNIH3T3 and TmNZB/B1NJ) contained 1.6- to 1.9-fold more mtDNA than those producing less ROS (TmC57BL/6J, TmCBA/J and TmBALB/cJ). In agreement with this, the complex I–deficient TmND6ko cells showed a more than threefold increase in mtDNA. The presence of NAC or Tiron was sufficient to reduce the amount of mtDNA in TmND6ko, TmNIH3T3 and TmNZB/B1NJ cells to that in TmC57BL/6J cells, but it did not substantially affect the amount of mtDNA in TmC57BL/6J, TmCBA/J and TmBALB/cJ (Fig. 2c, middle and right). These data are consistent with previous data associating mtDNA mutations with exacerbated ROS production and an increase in mtDNA copy number25,27,28. Consequently, they indicate that the respiration capacity per molecule of mtDNA may be lower in cells with the NIH3T3 or NZB mtDNA than in those with the C57BL/6J, CBA/J or BALB/cJ mtDNA and that the apparently normal level of respiration in all the transmitochondrial cells may be consequence of a compensatory ROS-mediated increase in mitochondrial biogenesis.
To test this possibility, we determined the effects of NAC and Tiron on the OXPHOS status of the different cell lines. One week of culture in the presence of NAC did not affect the oxygen consumption rate of the TmC57BL/6J, TmCBA/J or TmBALB/cJ cells (Fig. 1a, middle); however, the respiration capacity of both the TmNZB/B1NJ and TmNIH3T3 cells was significantly reduced. Similar results were obtained when the cells were grown in the presence of Tiron (Fig. 1a, right).
Differential effect of ROS scavengers on cybrid cell growth
NAC did not significantly modify the doubling time of any of the cells growing in glucose medium (Fig. 1c, middle); however, it did slow down the growth of all cell lines in galactose medium. Thus, the galactose/glucose growth ratio in the presence of NAC was 1.9; 2.3; 2.4 and 3.2 for TmC57BL/6J, TmCBA-BALB/c, TmNIH3T3 and TmNZB/B1NJ, respectively. By contrast, TmC57BL/6J and TmCBA-BALB/c cells were equally sensitive to the presence of Tiron in both media (galactose/glucose growth ratio 0.9 and 1, respectively). Again, although the effect of Tiron on the growth of TmNIH3T3 and TmNZB/B1NJ cells in glucose was similar to its effect on the other cell lines, this drug completely arrested TmNIH3T3 and TmNZB/B1NJ cell growth in galactose medium (Fig. 1c, right).
ROS signaling is required for cell adaptation to galactose
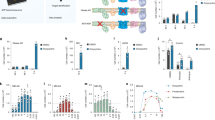
Because the above observations indicated that the signaling pathways that trigger the adaptation of energy metabolism when the cells are switched from glucose to galactose medium may be mediated by ROS, we assessed H2O2 production by the different cell lines in glucose versus galactose. The change in the carbon source induced a burst in the production of H2O2 by TmC57BL/6J and TmCBA-BALB/c cells, which could be seen after only 5 h (Fig. 3a). This production was maintained, albeit in lower amounts, after a week of continuous culturing in galactose. The increase in H2O2 production induced by the medium change was accompanied by an increase in the amount of mtDNA per cell (Fig. 3b) and maintenance of the respiratory rate (Fig. 3c) in TmC57BL/6J and TmCBA-BALB/c cells after 1 week of growth in galactose. Of note, TmNIH3T3 and TmNZB/B1NJ cells did not increase production of H2O2 in galactose as much as the other cells, probably because H2O2 production was already exacerbated in glucose in these cells (Fig. 3a). In agreement with this, growth of TmNIH3T3 and TmNZB/B1NJ cells in galactose did not induce a relevant increase in mtDNA (Fig. 3b); therefore, these cells did not achieve the oxygen consumption rate shown by TmC57BL/6J and TmCBA-BALB/c cells (Fig. 3c). The presence of NAC in galactose medium again prevented the burst in ROS and increase in mtDNA (Fig. 3b), and decreased further the respiration rate for TmNIH3T3 and TmNZB/B1NJ cells (Fig. 3c).
(a) Relative production of H2O2 in cells with different mtDNA haplotypes in glucose without drugs (GLU), after 5 h (GAL 5h) or 1 week (GAL 1W) in galactose without drugs, or after 1 week in galactose in the presence of NAC (GAL 1W + NAC). The production of H2O2 in TmC57BL/6J cells in glucose was set to 100%. Both the culturing conditions and the mtDNA haplotype induced significant differences in ROS production (ANOVA, P < 0.0001) and distinguished two groups of behavior with TmC57BL/6J and TmCBA-BALB/cJ in one group, and TmNIH3T3 and TmNZB/B1NJ in the other (see Supplementary Note). (b) mtDNA/nDNA ratio in transmitochondrial cybrids with different mtDNA haplotypes and under the indicated culturing conditions. Changes in mtDNA induced by the different incubation media without NAC were significant for TmC57BL/6J and TmCBA-BALB/c (P < 0.001) but not for TmNIH3T3 and TmNZB/B1NJ cells (see Supplementary Note). (c) Respiration rate in transmitochondrial cybrids with different mtDNA haplotypes under the indicated conditions. All culturing conditions except galactose for 5 h induced a significant difference in the respiration rate versus glucose (P < 0.05 for all paired cases), and a significant difference in the response of cells with different mtDNA haplotypes (P < 0.002) except for TmC57BL/6J versus TmCBA-BALB/cJ, and TmNIH3T3 versus TmNZB/B1NJ cells (see Supplementary Note). Data are mean ± s.d.
These observations strongly support the interpretation that mitochondria-generated ROS function as a regulatory signal that allows adjustment of the cellular OXPHOS capacity. Thus, the increase in mtDNA observed in TmNZB/B1NJ, TmNIH3T3 and TmND6ko cells when growing in glucose is a compensatory response induced by higher ROS production and aimed at increasing the OXPHOS capacity. This response in a wild-type background (NIH3T3 and NZB/B1NJ mtDNAs) is successful and sufficient to reach the appropriate respiration rate. This does not happen in complex I–deficient (TmND6ko) cells, even though the compensatory response is stronger, because the mtDNA is damaged in these cells.
Activity of TCA enzymes is modulated by mtDNA haplotype
Three of the above observations were difficult to interpret. First, the specific decrease in the glutamate plus malate RCR with no effect on the ADP/O ratio in TmNIH3T3 and TmNZB/B1NJ cells. Second, the apparently normal activity of the complex I–dependent respiration of TmNIH3T3 and TmNZB/B1NJ cells. Third, the decrease in the absolute rate of respiration of TmNIH3T3 and TmNZB/B1NJ cells when growing in galactose.
Inside the mitochondria, glutamate is transformed into α-ketoglutarate, which enters the tricarboxylic acid (TCA) cycle to produce NADH, initially as a substrate of α-ketoglutarate dehydrogenase (α-KGDH). It has been shown that α-KGDH is very sensitive to inhibition by ROS29,30. We therefore considered that, because TmNIH3T3 and TmNZB/B1NJ cells produce more ROS by the mitochondrial electron transport chain (mtETC), they might partially inhibit α-KGDH, in this way diminishing the rate of NADH generation, and thus the oxygen consumption rate, with substrates that feed electrons to complex I.
To address this possibility, we analyzed, in mitochondria from the different haplogroups, the activity of five different TCA cycle enzymes: citrate synthase, malate dehydrogenase (MDH), aconitase, α-KGDH and complex II (or succinate-CoQ oxidoreductase) together with the activity of complexes I and IV. The activity of complex I and complex II was slightly reduced in TmNIH3T3 and TmNZB/B1NJ cells, and the activity of complex IV was reduced only ∼20% in TmNZB/B1NJ cells, relative to TmC57BL/6J and TmCBA-BALB/c (Fig. 4). Thus, there were no substantial differences among the activity of the mtETC complexes induced by the mtDNA haplotype.
Shown is the relative enzymatic activity, normalized to citrate synthase, of enzymes of either the mtETC or the TCA cycle. The activity in cell line TmC57BL/6J was set to 100%. Asterisks indicate a significant difference versus TmC57BL/6J cells (see Supplementary Note). Data are mean ± s.d.
By contrast, the TCA cycle enzymes seemed to be influenced by the mtDNA haplotype in two ways. First, the activity of MDH was significantly increased in TmNIH3T3 and TmNZB/B1NJ mitochondria relative to TmC57BL/6J and TmCBA-BALB/c mitochondria. Second, the α-KGDH and aconitase relative activities were substantially decreased in TmNIH3T3 and TmNZB/B1NJ cells (Fig. 4). This observation indicates that the activity of α-KGDH and aconitase, enzymes known to be inhibited by ROS29,30,31,32, may be downregulated in those mitochondria with an mtDNA haplotype that enhances ROS production. In agreement with this interpretation, the relative activity of aconitase was recovered to that in TmC57BL/6J cells after TmNIH3T3 and TmNZB/B1NJ cells were grown in NAC (96.4 ± 6.0% and 102.7 ± 6.5% for TmNIH3T3 and TmNZB/B1NJ relative to TmC57BL/6J). By contrast, the MDH activity, which is ROS insensitive, remained unaffected. In addition, this downregulation offers a plausible explanation for the specific decrease in both the glutamate plus malate–dependent RCR and in the overall respiration rate in galactose shown by the different cells.
Key role of an 'A' track polymorphism in the mt-Tr gene
Although there are numerous sequence differences between NZB/B1NJ and C57BL/6J mtDNAs (Supplementary Table 1), there are only two differences between NIH3T3 and C57BL/6J mtDNAs: first, the insertion of two adenines at the DHU loop of the tRNAArg; and second, a synonymous polymorphism in the mt-Nd3 gene, the 9461T → C transition. Any potential role of the 9461T → C as the cause of the differences among TmC57BL/6J and TmNIH3T3 cells can be ruled out because BALB/cJ and CBA/J mtDNAs also have this polymorphism. In addition, the ND3 transcript level was found unchanged among the various haplotypes (Supplementary Fig. 3 and Supplementary Methods online).
Thus, there is only one potentially functional difference between NIH3T3 and C57BL/6J mtDNAs: the insertion of two adenines at the DHU loop of the tRNAArg. In addition, NZB/B1NJ mtDNA also carries this insertion of two adenines, whereas the BALB/cJ and CBA/J mtDNAs have a single adenine insertion (Table 1). Because none of the other nucleotide discrepancies between NZB/B1NJ and C57BL/6J mtDNAs is present in the NIH3T3 mtDNA, we speculate that the 'A' track polymorphism in the mt-Tr may be responsible for most, if not all, of the differences in ROS production, OXPHOS and TCA cycle performance and the differential response to NAC and Tiron.
Discussion
The contribution of the genetic background to health and disease and the identification of the genetic factors influencing drug response are research areas of paramount interest. The mtDNA hosts functionally relevant SNPs influencing health and disease. Although it has been reported that mtDNA variants predispose to disease1,8,11, to drug sensitivity33,34 and to more complex phenotypes such as learning performance14 or preferential accumulation in particular tissues13, subsequent biochemical and molecular analyses have concluded that the respiratory capacity of the cell is not affected by these mtDNA variations15,16. Here we challenge this conclusion by providing a conceptual framework to understand the associations between the different phenotypes observed and common mtDNA variants.
We have shown that two mtDNA variants that either predispose to poor learning capacities (NZB/B1NJ) or enhance the AHL phenotype (NIH3T3) have a respiratory performance apparently similar to that of other variants (C57BL/6J and CBA-BALB/c) when tested in a uniform nuclear background. Unexpectedly, this similarity in respiration capacity hides a genetically driven, intrinsic difference in the behavior of the mtETC, as revealed by differences in H2O2 production that correlate with a decrease in the activity of the ROS-sensitive enzymes aconitase and α-KGDH, and an increase in catalase activity. In addition, the effect of ROS scavengers (NAC and Tiron) on OXPHOS performance, mitochondrial biogenesis and growth rates in galactose validate these differences.
We identified the genetic element underlying these functional differences as an 'A' track polymorphism in the DHU loop of the tRNAArg. Thus, the cell lines with ten adenines have higher ROS production and increased amounts of mtDNA. The precise molecular mechanism by which the mt-Tr polymorphism leads to the observed differential performance of the mtETC is not known. It could be argued that the rate of mitochondrial protein synthesis is dependent on the mtDNA haplotype; however, it is difficult to envisage how a moderate quantitative difference in the rate of protein synthesis results in an increase in ROS production. Alternatively, the polymorphic A track in the tRNAArg DHU loop may influence translation fidelity rather than protein synthesis. If low quantities of polypeptides with missense mutations skip the protein quality control system and are incorporated into mtETC complexes, ROS would be induced at a higher rate, triggering the phenotypic consequences observed. Complex I would be more likely to be affected than any other complex because it contains 7 out of the 13 polypeptides encoded by mtDNA.
Numerous examples of tRNA mutations are strongly associated with human diseases for which a biochemical phenotype is difficult to assess35,36. The reasons for this apparent absence of biochemical phenotype remain obscure. The existence of inducible compensatory mechanisms such as those described here and the availability of drugs to block them are important developments that could help in such investigations.
Our work shows that generation of ROS by the mtETC is a fundamental mechanism of feedback signaling from the mitochondria to the nucleus that is used to adapt the OXPHOS capacity and, more generally, the bioenergetic metabolism to the cellular needs. Thus, the early stages of cell adaptation to an OXPHOS-demanding medium trigger ROS production and initiate an uncharacterized regulatory cascade aimed at adapting the cellular energetic metabolism. Our study also shows that key enzymes of the TCA cycle are influenced by the mtDNA haplotype, being reduced in those cells that generate more ROS. This reduction is specific to ROS-sensitive TCA cycle enzymes (α-KGDH and aconitase) and sets up a characteristic balance among TCA cycle enzymes induced by the mtDNA haplotype. This differential set up of the TCA cycle enzymes potentially has enormous consequences in important metabolic processes, other than ATP production, in which the cycle is involved. In addition, the different sensitivity to NAC and Tiron depending on the mtDNA haplotype indicates that mtDNA SNPs can induce distinct, and potentially undesired, responses to drugs of extended clinical use in humans, such as NAC.
As a whole, our results provide a molecular and conceptual framework to understand the role of mtDNA variants in predisposing to disease, in influencing cognitive capabilities and in the selective preference of some cell types for a given mtDNA haplotype. Because ROS function as cellular second messengers24,37 that broadly influence energetic metabolism, gene expression, cell proliferation and differentiation capabilities, an mtDNA-driven difference in ROS production should have profound influence in previously described phenotypes and very probably in others that have not been investigated as yet. In this respect, our findings for the mouse mtDNA haplotypes will help to understand the complex human phenotypes associated with common variants of mtDNA that lack a molecular explanation9,17.
Methods
Cell lines and media.
All cell lines were grown in Dulbecco's modified Eagle's medium (DMEM; GibcoBRL) supplemented with 5% fetal bovine serum (FBS; GibcoBRL). Where indicated, cells were grown for a week or for several hours in DMEM lacking glucose but containing 0.9 mg/ml of galactose supplemented with 5% dialyzed FBS (dFBS). The mtDNA-less cells used in this study (ρ°L929neo) were derived from L929 cells18 (ATCC CCL-1), a derivative clone of strain L generated from normal subcutaneous aerolar and adipose tissue of a 100-d-old C3H/An male mouse. ρ0L929neo cells were generated by long-term growth of the L929 mouse cell line in high concentrations of ethidium bromide and by transfection with the neo cassette–containing plasmid pcDNA3.1 (Invitrogen) as described18.
TmBALB/cJ, TmC57BL/6J and TmCBA/J were generated by transferring mitochondria from platelets to ρ0L929neo cells as described18,38. TmNIH3T3 and TmNZB/B1NJ cell lines were obtained by transformation of ρ0L929neo cells by cytoplast fusion39 using NIH3T3 fibroblasts or NZB/B1NJ primary hepatocytes as mitochondrial donors. The resulting 'transmitochondrial cybrids' were thus homogeneous for their nuclear genome but differed in their mtDNA haplotype, with both genomes being derived from the same mouse species (Mus musculus domesticus). Transmitochondrial cell lines were isolated by growing the cell population in DMEM supplemented with 5% dFBS and 500 μg/ml of Geneticin (G418, GibcoBRL).
TmND6ko cells were derived by random mutagenesis of NIH3T3 mouse cells as described20 and contain a deletion of one cytosine in a track of six cytosines at the mt-Nd6 gene. This deletion introduces a frameshift starting from amino acid 62 and creates a stop codon 32–34 bp downstream of the cytosine stretch, resulting in a truncated polypeptide of 72 amino acids, instead of the full-length ND6 protein of 172 amino acids. Where indicated, cells were grown for 7 d in the presence of 5 mM NAC (Sigma) or for 3 d in the presence of 1 mM Tiron (Fluka).
DNA analysis.
Total DNA from cell lines was extracted by using standard procedures. The complete mtDNA was amplified in 24 overlapping PCR fragments of 800–1,000 bp using a multifunctional robot40 (Genesis 150, Tecan). Primers were designed from the reference sequence22. Assembly and identification of variations in the mitochondrial DNA were carried out with the Staden package41.
Growth measurements.
Growth capacity in galactose-containing medium was determined by plating 5 × 104 cells on 12-well test plates in 2 ml of the appropriate medium (DMEM, which contains 4.5 mg/ml of glucose supplemented with 5% FBS, or DMEM lacking glucose but containing 0.9 mg/ml of galactose supplemented with 5% dFBS), incubating them at 37 °C for 5 d, and performing cell counts at daily intervals. Where indicated, cells were grown for 7 d in the presence of 5 mM NAC and were then plated (see above) to test their ability to grow in media containing glucose or galactose plus NAC. In the case of Tiron, cells were not pretreated with the drug before the growth measurement experiment; thus, cells were plated in media containing glucose or galactose plus Tiron.
Oxygen consumption measurements.
We carried out O2 consumption determinations in intact or in digitonin-permeabilized cells in an oxytherm Clark-type electrode (Hansatech) as described42 with small modifications18. The RCR and ADP/O ratio were determined in isolated mitochondria as described43.
Determination of H2O2 production.
Production of H2O2 was measured in cultured cells grown in the absence or presence of either NAC for 1 week or Tiron for 72 h as described44. In brief, 2′,7-dichlorodihydrofluorescein diacetate (2,7-DCFH2-DA; Fluka) to a final concentration of 100 μM was added to 100,000 cells, which were incubated at 37 °C for 30 min. The cells were then collected and the reaction was stopped by placing them in an ice bath for 5 min. The cells were disrupted by treatment with Triton X-100 (2%) and centrifuged at 2,500g for 20 min at 4 °C. The supernatant was used to measure fluorescence emission (excitation, 485 nm; emission, 535 nm) in a Spectrafluor plus instrument (Tecan). The amount of H2O2 produced was calculated by using a standard curve of 2,7-DCFH2 in which 1 μM of 2,7-DCFH2 represented 1 μM of H2O2.
Mitochondrial DNA copy number quantification.
We quantified mtDNA by real-time PCR using an ABI PRISM 7000 Sequence Detector System (Applied Biosystems) and Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). Total cellular DNA was used as a template and was amplified with specific oligodeoxynucleotides for mt-Co2 and Sdha (Supplementary Table 2 online). These primers were designed with Primer Express 2.0 software (Applied Biosystems). They were tested functionally in quantitative analysis and the results were optimal. Melting curve analysis showed that there was no primer dimer formation.
Real-time PCR amplification was done in accordance with the manufacturer's recommendations in a final volume of 25 μl using ROX as the reference dye. The final concentrations of primers and MgCl2 were 0.2 μM and 3 mM, respectively. We calculated the mtDNA copy number per cell using Sdha amplification as a reference for nuclear DNA content.
Catalase activity measurement.
Enzymatic activities were measured with a UV 500 spectrophotometer (Unicam). Catalase activity was assayed in total cellular homogenate by a published method45, in which the absorbance decrease at 240 nm was measured in a medium containing 20 mM H2O2 and 10 mM potassium phosphate buffer (pH 7). One unit of the enzyme was defined as 1 μmol of H2O2 consumed per minute. Specific activity is reported as units per milligram of protein estimated by the Bradford assay46.
TCA cycle enzyme activity measurements.
Mitochondria were isolated as described40 and the different enzymatic activities were assessed by spectrophotometry. Aconitase activity was assayed by following the linear increase in absorbance at 340 nm at 25 °C in a reaction mixture containing 50 mM Tris-HCl (pH 7.4), 5 mM sodium citrate, 0.2% Triton X-100, 0.6 mM MnCl2, 0.2 mM NADP+, 2 U of NADP-dependent isocitrate dehydrogenase and 100 μg of mitochondrial protein31. α-KGDH activity was measured by following the linear change in absorbance at 340 nm in a reaction mixture containing 50 mM Tris-HCl (pH 7.0), 0.1% Triton X-100, 0.2 mM NAD+, 1 mM α-ketoglutarate, 50 μM coenzyme A, 0.5 mM KCN and 10 μM cocarboxilase incubated at 25 °C (ref. 31). MDH activity was measured as described30 by following the linear decrease in absorbance at 340 nm in a reaction medium containing 10 μM rotenone, 0.2% Triton X-100, 0.15 mM NADH and 100 mM potassium phosphate buffer (pH 7.4) at 37 °C. The reaction was started by the addition of 0.33 mM oxaloacetate. The activities of citrate synthase and complexes I, II and IV were measured in isolated mitochondria as described20.
Mitochondrial RNA isolation.
The mitochondrial fraction, isolated from cell cultures as described43, was suspended in 10 mM Tris-HCl (pH 7.4), 0.15 M NaCl and 1 mM EDTA and incubated for 15 min at 37 °C in the presence of proteinase k (200 μg/ml), SDS (2%) and RNase-free DNase (Roche). Total mitochondrial RNAs were then extracted with an equal volume of phenol/chloroform/isoamyl alcohol (25/25/1 v/v/v) and precipitated with ethanol47.
Quantification of the mitochondrial tRNAArg.
The relative content of mitochondrial tRNAArg was determined as described48. In brief, total mitochondrial RNA samples were resolved by electrophoresis through a 10% polyacrylamide and 7 M urea gel in Tris-borate-EDTA buffer (after heating the sample at 70 °C for 10 min) and then electroblotted onto a Zeta-probe membrane (Bio-Rad) for hybridization analysis with specific oligodeoxynucleotides probes. These probes were 5′-end-labeled with T4 polynucleotide kinase (Promega) using [γ32P]dATP (Amersham). To detect mitochondrial tRNAArg, tRNAGly and tRNAHis, oligodeoxynucleotides specific for each tRNA were used (Supplementary Table 2).
The hybridization reactions were carried out in a mixture of 6 × SSC, 0.1% sodium pyrophosphate, 5 × Denhardt's solution, 0.1% SDS and 250 μg/ml of salmon sperm DNA for 4 h at 37 °C. After hybridization, the membranes were washed twice for 10 min in 2 × SSC plus 0.1% SDS at 37 °C.
Statistical analysis.
Differences between cell lines carrying particular mtDNA haplotypes for the various parameters analyzed were assessed by ANOVA and by the nonparametric Kruskal-Wallis test. Paired haplotype differences were assessed by the post-hoc Fisher's PLSD test. All test and calculations were done with the statistical package StatView 5.0 for Macintosh (SAS Institute).
Accession codes.
GenBank: complete sequences for mitochondrial genomes: C57BL6/J (NC_005089)22, CBA/J (AY46649), BALB/cJ (AJ512208)22, NIH/3T3 (AY999076), NZB/B1NJ (L07095)21 and L929 (AJ489607)22.
Note: Supplementary information is available on the Nature Genetics website.
References
Ruiz-Pesini, E. et al. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 67, 682–696 (2000).
Montiel-Sosa, F. et al. Differences of sperm motility in mitochondrial DNA haplogroup U sublineages. Gene 368C, 21–27 (2006).
Tanaka, M., Gong, J.S., Zhang, J., Yoneda, M. & Yagi, K. Mitochondrial genotype associated with longevity. Lancet 351, 185–186 (1998).
De Benedictis, G. et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J. 13, 1532–1536 (1999).
Niemi, A.K. et al. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum. Genet. 112, 29–33 (2003).
Brown, M.D. et al. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup J. Hum. Genet. 110, 130–138 (2002).
Howell, N., Herrnstadt, C., Shults, C. & Mackey, D.A. Low penetrance of the 14484 LHON mutation when it arises in a non-haplogroup J mtDNA background. Am. J. Med. Genet. A. 119, 147–151 (2003).
Wallace, D.C., Ruiz-Pesini, E. & Mishmar, D. mtDNA variation, climatic adaptation, degenerative diseases, and longevity. Cold Spring Harb. Symp. Quant. Biol. 68, 479–486 (2003).
Dimauro, S. Mitochondrial medicine. Biochim. Biophys. Acta 1659, 107–114 (2004).
Ruiz-Pesini, E., Mishmar, D., Brandon, M., Procaccio, V. & Wallace, D.C. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226 (2004).
Johnson, K.R., Zheng, Q.Y., Bykhovskaya, Y., Spirina, O. & Fischel-Ghodsian, N. A nuclear–mitochondrial DNA interaction affecting hearing impairment in mice. Nat. Genet. 27, 191–194 (2001).
Noben-Trauth, K., Zheng, Q.Y. & Johnson, K.R. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 35, 21–23 (2003).
Jenuth, J.P., Peterson, A.C. & Shoubridge, E.A. Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat. Genet. 16, 93–95 (1997).
Roubertoux, P.L. et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat. Genet. 35, 65–69 (2003).
Carelli, V. et al. Respiratory function in cybrid cell lines carrying European mtDNA haplogroups: implications for Leber's hereditary optic neuropathy. Biochim. Biophys. Acta 1588, 7–14 (2002).
Battersby, B.J. & Shoubridge, E.A. Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum. Mol. Genet. 10, 2469–2479 (2001).
Herrnstadt, C. & Howell, N. An evolutionary perspective on pathogenic mtDNA mutations: haplogroup associations of clinical disorders. Mitochondrion 4, 797–798 (2004).
Acin-Perez, R. et al. An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum. Mol. Genet. 12, 329–339 (2003).
Bai, Y. & Attardi, G. The mtDNA-encoded ND6 subunit of mitochondrial NADH dehydrogenase is essential for the assembly of the membrane arm and the respiratory function of the enzyme. EMBO J. 17, 4848–4858 (1998).
Acin-Perez, R. et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol. Cell 13, 805–815 (2004).
Loveland, B., Wang, C.R., Yonekawa, H., Hermel, E. & Lindahl, K.F. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell 60, 971–980 (1990).
Bayona-Bafaluy, M.P. et al. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 31, 5349–5355 (2003).
Beckman, K.B. & Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Lee, H.C. & Wei, Y.H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 37, 822–834 (2005).
Geromel, V. et al. Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum. Mol. Genet. 10, 1221–1228 (2001).
Lee, H.C., Yin, P.H., Chi, C.W. & Wei, Y.H. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J. Biomed. Sci. 9, 517–526 (2002).
Liu, C.S. et al. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic. Res. 37, 1307–1317 (2003).
Nulton-Persson, A.C. & Szweda, L.I. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 276, 23357–23361 (2001).
Tretter, L. & Adam-Vizi, V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of α-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 20, 8972–8979 (2000).
Gardner, P.R., Nguyen, D.D. & White, C.W. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Natl. Acad. Sci. USA 91, 12248–12252 (1994).
Gardner, P.R., Raineri, I., Epstein, L.B. & White, C.W. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 270, 13399–13405 (1995).
Zhao, H. et al. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am. J. Hum. Genet. 74, 139–152 (2004).
Prezant, T.R. et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 4, 289–294 (1993).
Reid, F.M., Vernham, G.A. & Jacobs, H.T. A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum. Mutat. 3, 243–247 (1994).
Guan, M.X. et al. The deafness-associated mitochondrial DNA mutation at position 7445, which affects tRNASerUCN precursor processing, has long-range effects on NADH dehydrogenase subunit ND6 gene expression. Mol. Cell. Biol. 18, 5868–5879 (1998).
Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 (2003).
Chomyn, A. et al. Platelet-mediated transformation of mtDNA-less human cells: analysis of phenotypic variability among clones from normal individuals—and complementation behavior of the tRNALys mutation causing myoclonic epilepsy and ragged red fibers. Am. J. Hum. Genet. 54, 966–974 (1994).
King, M.P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
Gallardo, M.E. et al. m627G>A, a recurrent mutation in the human mitochondrial DNA that reduce cytochrome c oxidase activity and is associated with tumors. Hum. Mutat. 27, 575–582 (2006).
Staden, R., Beal, K.F. & Bonfield, J.K. The Staden package, 1998. Methods Mol. Biol. 132, 115–130 (2000).
Hofhaus, G., Shakeley, R.M. & Attardi, G. Use of polarography to detect respiration defects in cell cultures. Methods Enzymol. 264, 476–483 (1996).
Fernandez-Vizarra, E., Lopez-Perez, M.J. & Enriquez, J.A. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods 26, 292–297 (2002).
Wang, H. & Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 27, 612–616 (1999).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Enriquez, J.A., Fernandez-Silva, P., Perez-Martos, A., Lopez-Perez, M.J. & Montoya, J. The synthesis of mRNA in isolated mitochondria can be maintained for several hours and is inhibited by high levels of ATP. Eur. J. Biochem. 237, 601–610 (1996).
Enriquez, J.A., Chomyn, A. & Attardi, G. MtDNA mutation in MERRF syndrome causes defective aminoacylation of tRNALys and premature translation termination. Nat. Genet. 10, 47–55 (1995).
Acknowledgements
We thank C. Moraes and E. Shoubridge for the NZB hepatocytes; E. Ruiz-Pesini, A. Barrientos, C. López-Otin and M. Palacín for comments on the manuscript; and S. Morales for technical assistance. Our work was supported by the Spanish Ministry of Education (SAF2003-00103), the Instituto de Salud Carlos III (REDEMETH-G03/054, REDCIEN C03/06-Grupo RC-N34-3 yr ECEMECRE G03/011), the European Union (EUMITOCOMBAT-LSHM-CT-2004-503116), Group of Excellence grant DGA (B55) and Fundación Ramón Areces. R.M.-L. and R.A.-P. are supported by a predoctoral fellowship from the Spanish Ministry of Education and M.E.G. is supported by an I3P postdoctoral contract from the CSIC, Spain.
Author information
Authors and Affiliations
Contributions
This study was designed and the paper was written by P.F.-S., A.P.-M., S.R.d.C. and J.A.E.; the wild-type cybrid cell line was constructed and phenotype assessed by R.M.-L. and R.A.-P.; the TmND6ko mtDNA mutant cell line was generated and characterized by N.M.; and the mtDNA resequencing strategy was designed by, and full mtDNA sequencing performed by, M.E.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
RFLP analysis to detect the possible presence of L929 mtDNA in the transmitochondrial cell lines. (PDF 112 kb)
Supplementary Fig. 2
Protein levels for specific subunits of the mtETC complexes in the different mtDNA haplotypes. (PDF 104 kb)
Supplementary Fig. 3
ND3/ND4 relative transcript levels in the transmitochondrial cell lines. (PDF 98 kb)
Supplementary Table 1
mtDNA nucleotide differences between C57BL/6J and NZB/B1NJ. (PDF 18 kb)
Supplementary Table 2
Primer sequences. (PDF 80 kb)
Rights and permissions
About this article
Cite this article
Moreno-Loshuertos, R., Acín-Pérez, R., Fernández-Silva, P. et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet 38, 1261–1268 (2006). https://doi.org/10.1038/ng1897
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1897
This article is cited by
-
Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2
Nature Metabolism (2021)
-
Presence of male mitochondria in somatic tissues and their functional importance at the whole animal level in the marine bivalve Arctica islandica
Communications Biology (2021)
-
Common mtDNA variations at C5178a and A249d/T6392C/G10310A decrease the risk of severe COVID-19 in a Han Chinese population from Central China
Military Medical Research (2021)
-
ROS and diseases: role in metabolism and energy supply
Molecular and Cellular Biochemistry (2020)
-
Novel compound mutations in the mitochondrial translation elongation factor (TSFM) gene cause severe cardiomyopathy with myocardial fibro-adipose replacement
Scientific Reports (2019)