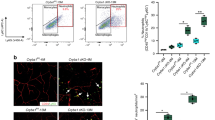
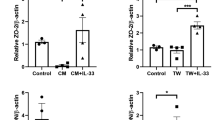
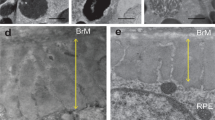
Abstract
Age-related macular degeneration (AMD) is the leading cause of central vision loss worldwide. Drusen accumulation is the major pathological hallmark common to both dry and wet AMD. Although activation of the immune system has been implicated in disease progression, the pathways involved are unclear. Here we show that drusen isolated from donor AMD eyes activates the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome, causing secretion of interleukin-1β (IL-1β) and IL-18. Drusen component C1Q also activates the NLRP3 inflammasome. Moreover, the oxidative-stress–related protein-modification carboxyethylpyrrole (CEP), a biomarker of AMD, primes the inflammasome. We found cleaved caspase-1 and NLRP3 in activated macrophages in the retinas of mice immunized with CEP-adducted mouse serum albumin, modeling a dry-AMD–like pathology. We show that laser-induced choroidal neovascularization (CNV), a mouse model of wet AMD, is exacerbated in Nlrp3−/− but not Il1r1−/− mice, directly implicating IL-18 in the regulation of CNV development. These findings indicate a protective role for NLRP3 and IL-18 in the progression of AMD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Swaroop, A., Chew, E.Y., Rickman, C.B. & Abecasis, G.R. Unravelling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu. Rev. Genomics Hum. Genet. 10, 19–43 (2009).
Resnikoff, S. et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 82, 844–851 (2004).
Bressler, S.B., Maguire, M.G., Bressler, N.M. & Fine, S.L. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch. Ophthalmol. 108, 1442–1447 (1990).
Sarks, S.H., van Driel, D., Maxwell, L. & Killingsworth, M. Softening of drusen and subretinal neovascularization. Trans. Ophthalmol. Soc. U.K. 100, 414–422 (1980).
Vinding, T. Occurrence of drusen, pigmentary changes, and exudative changes in the macula with reference to age-related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol. (Copenh.) 68, 410–414 (1990).
Holz, F.G., Bellman, C., Staudt, S., Schutt, F. & Volcker, H.E. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1051–1056 (2001).
Seddon, J.M., George, S. & Rosner, B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 124, 995–1001 (2006).
Edwards, A.O. & Malek, G. Molecular genetics of AMD and current animal models. Angiogenesis 10, 119–132 (2007).
Canter, J.A. et al. Mitochondrial DNA polymorphism A4917G is independently associated with age-related macular degeneration. PLoS ONE 3, e2091 (2008).
Chen, Y., Bedell, M. & Zhang, K. Age-related macular degeneration: genetic and environmental factors of disease. Mol. Interv. 10, 271–281 (2010).
Leveziel, N. et al. Genetic factors associated with age-related macular degeneration. Ophthalmologica 226, 87–102 (2011).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008).
Zhou, R., Yazdi, A.S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Gao, H. & Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 33, 1–17 (1992).
Iyer, S.S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 20388–20393 (2009).
Hollyfield, J.G. et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 14, 194–198 (2008).
Gu, X. et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 278, 42027–42035 (2003).
Sakurai, E. et al. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 44, 3578–3585 (2003).
Campbell, M. et al. Systemic low molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol. Med. 3, 235–245 (2011).
Ma, W., Zhao, L., Fontainhas, A.M., Fariss, R.N. & Wong, W.T. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS ONE 4, e7945 (2009).
Anderson, D.H. et al. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 78, 243–256 (2004).
Luibl, V. et al. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J. Clin. Invest. 116, 378–385 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Lin, H. et al. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 3521–3529 (2011).
Wang, A.L. et al. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy 5, 563–564 (2009).
Zarbin, M.A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 122, 598–614 (2004).
West, X.Z. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467, 972–976 (2010).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Hageman, G.S. & Mullins, R.F. Molecular composition of drusen as related to substructural phenotype. Mol. Vis. 5, 28–(1999).
Nauta, A.J. et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 32, 1726–1736 (2002).
Blanquet-Grossard, F., Thielens, N.M., Vendrely, C., Jamin, M. & Arlaud, G.J. Complement protein C1q recognizes a conformationally modified form of the prion protein. Biochemistry 44, 4349–4356 (2005).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Luo, X., Weber, G.A., Zheng, J., Gendelman, H.E. & Ikezu, T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer's disease. J. Neuroimmunol. 135, 62–71 (2003).
Ten, V.S. et al. Complement component c1q mediates mitochondria-driven oxidative stress in neonatal hypoxic-ischemic brain injury. J. Neurosci. 30, 2077–2087 (2010).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
CATT Research Group. et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897–1908 (2011).
Crabb, J.W. et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 99, 14682–14687 (2002).
Ishii, T., Haga, S., Yagishita, S. & Tateishi, J. The presence of complements in amyloid plaques of Creutzfeldt-Jakob disease and Gerstmann-Straussler-Scheinker disease. Appl. Pathol. 2, 370–379 (1984).
Xu, H., Chen, M. & Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 28, 348–368 (2009).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Arias, L. et al. Intravitreal infliximab in patients with macular degeneration who are nonresponders to antivascular endothelial growth factor therapy. Retina 30, 1601–1608 (2010).
Giganti, M. et al. Adverse events after intravitreal infliximab (Remicade). Retina 30, 71–80 (2010).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Allen, I.C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Qiao, H. et al. Abnormal retinal vascular development in IL-18 knockout mice. Lab. Invest. 84, 973–980 (2004).
Qiao, H. et al. Interleukin-18 regulates pathological intraocular neovascularization. J. Leukoc. Biol. 81, 1012–1021 (2007).
Mallat, Z. et al. Interleukin-18/interleukin-18 binding protein signaling modulates ischemia-induced neovascularization in mice hindlimb. Circ. Res. 91, 441–448 (2002).
Coughlin, C.M. et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J. Clin. Invest. 101, 1441–1452 (1998).
Jiang, H.R. et al. IL-18 not required for IRBP peptide-induced EAU: studies in gene-deficient mice. Invest. Ophthalmol. Vis. Sci. 42, 177–182 (2001).
Gu, X. et al. Oxidatively truncated docosahexaenoate phospholipids: total synthesis, generation, and peptide adduction chemistry. J. Org. Chem. 68, 3749–3761 (2003).
Percopo, C.M., Hooks, J.J., Shinohara, T., Caspi, R. & Detrick, B. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J. Immunol. 145, 4101–4107 (1990).
Marmor, M.F. et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol. 1, 69–77 (2009).
Acknowledgements
The Ocular Genetics Unit at Trinity College Dublin is supported by the American Health Assistance Foundation (AHAF), Science Foundation Ireland (SFI), The Health Research Board of Ireland (HRB), Irish Research Council for Science Engineering and Technology (IRCSET), Enterprise Ireland (EI), Telemedicine and Advanced Technology Research Center (TATRC) and Fighting Blindness Ireland (FB-Ireland). S.L.D. and L.A.J.O. are supported by the SFI Immunology Research Centre (IRC) and the SFI Strategic Research Cluster (07/SRC/B1144). Support for the laboratory of J.G.H. was provided by the US National Institutes of Health through grant EY014240, the Macular Vision Research Foundation, Research to Prevent Blindness and the Llura and Gordon Gund Foundation. Support for the laboratory of R.G.S. was provided by the US National Institutes of Health through grants GM021249 and EY016813. Support for the laboratory of E.C.L. was from SFI grant number 08/RFP/MBT1363 and SFI Strategic Research Cluster 07/SRC/B1144. We would like to thank C. Woods, C. Murray, D. Flynn and R. Robertson for animal husbandry. The authors would also like to thank the Research Foundation at the Royal Victoria Eye and Ear Hospital for assistance in the acquisition of the Iridex laser system. We thank E. Latz (University of Bonn) for the provision of BMDMs expressing YFP-labeled ASC. We would like to acknowledge the expertise of K. Shadrach in isolating drusen from AMD donor eyes.
Author information
Authors and Affiliations
Contributions
S.L.D. and M.C. conceived of, designed and performed experiments and wrote the paper. E.O. performed experiments. R.G.S. prepared the CEP-HSA. A.M. genotyped all animals reported in the paper and isolated cells. P.F.K. performed ERG analyses. G.J.F., A.-S.K. and M.M.H. contributed to analysis of experiments and data. E.C.L. and L.A.J.O. conceived of experiments and analyzed data. J.G.H. isolated drusen and wrote the paper. P.H. conceived of and designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–14 (PDF 1241 kb)
Rights and permissions
About this article
Cite this article
Doyle, S., Campbell, M., Ozaki, E. et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 18, 791–798 (2012). https://doi.org/10.1038/nm.2717
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2717
This article is cited by
-
Recent Insights in Pyrin Inflammasome Activation: Identifying Potential Novel Therapeutic Approaches in Pyrin-Associated Autoinflammatory Syndromes
Journal of Clinical Immunology (2024)
-
Impairing Gasdermin D-mediated pyroptosis is protective against retinal degeneration
Journal of Neuroinflammation (2023)
-
Association of NLRPs with pathogenesis of dry age-related macular degeneration
International Ophthalmology (2023)
-
Ocular surface toll like receptors in ageing
BMC Ophthalmology (2022)
-
Spotlight on pyroptosis: role in pathogenesis and therapeutic potential of ocular diseases
Journal of Neuroinflammation (2022)