Abstract
Over 20% of the drugs for treating human diseases target ion channels, but no cancer drug approved by the US Food and Drug Administration (FDA) is intended to target an ion channel. We found that the EAG2 (Ether-a-go-go 2) potassium channel has an evolutionarily conserved function for promoting brain tumor growth and metastasis, delineate downstream pathways, and uncover a mechanism for different potassium channels to functionally cooperate and regulate mitotic cell volume and tumor progression. EAG2 potassium channel was enriched at the trailing edge of migrating medulloblastoma (MB) cells to regulate local cell volume dynamics, thereby facilitating cell motility. We identified the FDA-approved antipsychotic drug thioridazine as an EAG2 channel blocker that reduces xenografted MB growth and metastasis, and present a case report of repurposing thioridazine for treating a human patient. Our findings illustrate the potential of targeting ion channels in cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Thompson, M.C. et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J. Clin. Oncol. 24, 1924–1931 (2006).
Kool, M. et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE 3, e3088 (2008).
Cho, Y.J. et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 29, 1424–1430 (2011).
Northcott, P.A. et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 29, 1408–1414 (2011).
Goodrich, L.V., Milenkovic, L., Higgins, K.M. & Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Yang, Z.J. et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 14, 135–145 (2008).
Schüller, U. et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134 (2008).
Hatton, B.A. et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 68, 1768–1776 (2008).
Romer, J. & Curran, T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 65, 4975–4978 (2005).
Rudin, C.M. et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 361, 1173–1178 (2009).
Yauch, R.L. et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326, 572–574 (2009).
Wu, X. et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature 482, 529–533 (2012).
Schönherr, R. Clinical relevance of ion channels for diagnosis and therapy of cancer. J. Membr. Biol. 205, 175–184 (2005).
Pardo, L.A. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 19, 285–292 (2004).
Fraser, S.P. & Pardo, L.A. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on ion channels and cancer. EMBO Rep. 9, 512–515 (2008).
Prevarskaya, N., Skryma, R. & Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 16, 107–121 (2010).
Huang, X. & Jan, L.Y. Targeting potassium channels in cancer. J. Cell Biol. 206, 151–162 (2014).
Homem, C.C. & Knoblich, J.A. Drosophila neuroblasts: a model for stem cell biology. Development 139, 4297–4310 (2012).
Zhu, S. et al. The bHLH repressor Deadpan regulates the self-renewal and specification of Drosophila larval neural stem cells independently of Notch. PLoS ONE 7, e46724 (2012).
Bowman, S.K. et al. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev. Cell 14, 535–546 (2008).
Richter, C., Oktaba, K., Steinmann, J., Muller, J. & Knoblich, J.A. The tumour suppressor L(3)mbt inhibits neuroepithelial proliferation and acts on insulator elements. Nat. Cell Biol. 13, 1029–1039 (2011).
Northcott, P.A. et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat. Genet. 41, 465–472 (2009).
Gonzalez, C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 13, 172–183 (2013).
Huang, X. et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev. 26, 1780–1796 (2012).
Saini, N. & Reichert, H. Neural stem cells in Drosophila: molecular genetic mechanisms underlying normal neural proliferation and abnormal brain tumor formation. Stem Cells Int. 2012, 486169 (2012).
Reimand, J., Arak, T. & Vilo, J. g:Profiler–a web server for functional interpretation of gene lists (2011 update). Nucleic Acids Res. 39, W307–W315 (2011).
Merico, D., Isserlin, R., Stueker, O., Emili, A. & Bader, G.D. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5, e13984 (2010).
Bhattacharjee, A. et al. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci. 23, 11681–11691 (2003).
Kaczmarek, L.K. Slack, Slick and sodium-activated potassium channels. ISRN Neurosci. 2013, 354262 (2013).
Hage, T.A. & Salkoff, L. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J. Neurosci. 32, 2714–2721 (2012).
Rutkowski, S. et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J. Clin. Oncol. 28, 4961–4968 (2010).
García-Ferreiro, R.E. et al. Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J. Gen. Physiol. 124, 301–317 (2004).
Herzberg, I.M., Trudeau, M.C. & Robertson, G.A. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J. Physiol. (Lond.) 511, 3–14 (1998).
Preskorn, S.H. Antipsychotic drug development in the pre-human-genome era: a full circle. J. Psychiatr. Pract. 7, 209–213 (2001).
Vanderheeren, F.A. & Muusze, R.G. Plasma levels and half lives of thioridazine and some of its metabolites. I. High doses in young acute schizophrenics. Eur. J. Clin. Pharmacol. 11, 135–140 (1977).
Svendsen, C.N., Hrbek, C.C., Casendino, M., Nichols, R.D. & Bird, E.D. Concentration and distribution of thioridazine and metabolites in schizophrenic post-mortem brain tissue. Psychiatry Res. 23, 1–10 (1988).
Willoughby, L.F. et al. An in vivo large-scale chemical screening platform using Drosophila for anti-cancer drug discovery. Dis. Model. Mech. 6, 521–529 (2013).
Habela, C.W. & Sontheimer, H. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle 6, 1613–1620 (2007).
Boucrot, E. & Kirchhausen, T. Mammalian cells change volume during mitosis. PLoS ONE 3, e1477 (2008).
Schwab, A., Fabian, A., Hanley, P.J. & Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913 (2012).
Jakab, M. & Ritter, M. Cell volume regulatory ion transport in the regulation of cell migration. Contrib. Nephrol. 152, 161–180 (2006).
Schwab, A., Hanley, P., Fabian, A. & Stock, C. Potassium channels keep mobile cells on the go. Physiology (Bethesda) 23, 212–220 (2008).
Schwab, A. et al. Polarized ion transport during migration of transformed Madin-Darby canine kidney cells. Pflugers Arch. 430, 802–807 (1995).
Schneider, S.W. et al. Volume dynamics in migrating epithelial cells measured with atomic force microscopy. Pflugers Arch. 439, 297–303 (2000).
Schwab, A. et al. Subcellular distribution of calcium-sensitive potassium channels (IK1) in migrating cells. J. Cell. Physiol. 206, 86–94 (2006).
Ward, R.J. et al. Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res. 69, 4682–4690 (2009).
Huang, X., Ketova, T., Litingtung, Y. & Chiang, C. Isolation, enrichment, and maintenance of medulloblastoma stem cells. J. Vis. Exp. published online, 2086 (1 September 2010).
Berman, D.M. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561 (2002).
Corno, D. et al. Gene signatures associated with mouse postnatal hindbrain neural stem cells and medulloblastoma cancer stem cells identify novel molecular mediators and predict human medulloblastoma molecular classification. Cancer Discov. 2, 554–568 (2012).
Lee, H.Y., Greene, L.A., Mason, C.A. & Manzini, M.C. Isolation and culture of post-natal mouse cerebellar granule neuron progenitor cells and neurons. J. Vis. Exp. published online, doi:10.3791/99010.3791/990 (16 January 2009).
Wallace, K., Liu, T.H. & Vaessin, H. The pan-neural bHLH proteins DEADPAN and ASENSE regulate mitotic activity and cdk inhibitor dacapo expression in the Drosophila larval optic lobes. Genesis 26, 77–85 (2000).
Wang, Z., Wilson, G.F. & Griffith, L.C. Calcium/calmodulin-dependent protein kinase II phosphorylates and regulates the Drosophila eag potassium channel. J. Biol. Chem. 277, 24022–24029 (2002).
Broughton, S.J., Kitamoto, T. & Greenspan, R.J. Excitatory and inhibitory switches for courtship in the brain of Drosophila melanogaster. Curr. Biol. 14, 538–547 (2004).
Vahasoyrinki, M., Niven, J.E., Hardie, R.C., Weckstrom, M. & Juusola, M. Robustness of neural coding in Drosophila photoreceptors in the absence of slow delayed rectifier K+ channels. J. Neurosci. 26, 2652–2660 (2006).
Janic, A., Mendizabal, L., Llamazares, S., Rossell, D. & Gonzalez, C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 330, 1824–1827 (2010).
Acknowledgements
We thank Y. Song, W.-P. Ge, S.-B. Yang, H. Yang, C. Peters, B. Piggott, S. Morrissy, D. Shih, B.K. Shoichet and all of the members of the Jan laboratory for constructive suggestions. We thank C. Chiang for critical reading of the manuscript. This work was supported by the GEMS-CTSI postdoctoral award from the Howard Hughes Medical Institute and UCSF, and the Damon Runyon Cancer Research Foundation Fellowship to X.H. (Kandis Ann Ulrich-Carleton Fellow), NIH grant P41 GM103504 to G.D.B., the Pediatric Brain Tumor Foundation and R01 grants CA133091, CA148699 and CA159859 to W.A.W. and M.D.T., the Garron Family Chair in Childhood Cancer Research at The Hospital for Sick Children and The University of Toronto, operating funds from the Canadian Institutes of Health Research, the Terry Fox Research Institute, and the Pediatric Brain Tumor Foundation to M.D.T., NIH grants R37NS040929 to Y.N.J., and R37MH065334 and R01CA185039 to L.Y.J. Y.N.J. and L.Y.J. are Howard Hughes Medical Institute investigators.
Author information
Authors and Affiliations
Contributions
X.H. and L.Y.J. conceived and designed the study. X.H., Y.H., A.M.D., R.H., W.Z., J.R., H.Y., T.A.W., S.J.S., S.Y., S.B., S.Z., M.K.C., J.P., V.R., L.G., X.W., M.R., C.M.F., C.C.K., G.D.B. and S.M. contributed to methodology and data acquisition. X.H., Y.H., A.M.D., W.Z., J.R., S.Y., J.P., V.R., C.M.F., C.C.K., W.A.W., C.D.J., M.A.S., G.D.B., S.M., M.D.T., Y.N.J. and L.Y.J. analyzed and interpreted the data. X.H., Y.N.J. and L.Y.J. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 eag loss-of-function mutation reduces the growth of brain tumors induced by reduction of tumor suppressor Brat activity, and transplantation assay to study Drosophila brain tumor metastasis
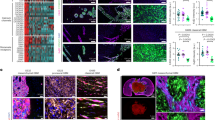
a, External view of the control brains, insc-Gal4-driven bratRNAi-induced brain tumors and bratRNAi-induced brain tumors with eag loss-of-function (eag1) at 25oC (n = 9 and 8 for bratRNAi and bratRNAi; eag1, respectively, P = 0.0002, two-tailed Student’s t-test) and 29oC (n = 10 and 10 for bratRNAi and bratRNAi; eag1, respectively, P < 0.0001, two-tailed Student’s t-test). b, GFP-labeled, dpn-overexpressing primary brain lobe tumors were retrieved from 3rd instar larvae. The tumors were micro-dissected into small pieces with equal volumes. Young female adult hosts were anesthetized with CO2 and a piece of larval brain was picked up with the tip of a glass needle and injected tangentially into the mid-ventral part of the abdomen. Implanted hosts were kept at 25°C for 10 days and monitored for tumor growth in the abdomen and metastasis into the brain. Tumor formation or metastasis was detected in hosts transplanted with dpn-overexpressing tumor cells, and the tumor-transplanted hosts displayed significantly shortened survival compared to the hosts transplanted with normal brain cells retrieved from w- control larvae (n = 12 and 20 for control and dpnO/E, respectively, log-rank Kaplan-Meier test).
Supplementary Figure 2 Mutation status of KCNT2 across different cancer types and analysis of KCNT2 knockdown in MB cells
a, Analysis of large-scale cancer genomics datasets shows frequent KCNT2 gene amplification and mutations in different cancer types. Data analysis and visualization are performed using the cBioPortal Cancer Genomics website (http://www.cbioportal.org). b, Lentivirus-mediated RNAi knockdown of KCNT2 reduces the clonogenic growth of MB cells. c, KCNT2 RNAi treatment in MB cells significantly reduces KCNT2 transcript level (n = 3 independent cultures and qPCR experiments, two-tailed Student’s t-test). Error bars show ± SEM.
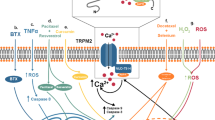
Supplementary Figure 3 Working model: co-option of potassium channel activity regulates mitotic cell volume in MB
Left: Potassium channel activity is increased in MB cells during late G2 and mitotic phases, which regulates cell volume reduction. Right: EAG2, which localizes to the plasma membrane at late G2 and during mitosis, regulates KCNT2 mRNA expression. KCNT2 channel enriches to the plasma membrane beginning at metaphase to cooperate with EAG2 and regulate mitotic volume dynamics. EAG2 channel block by astemizole or thioridazine, or indirect inhibition of KCNT2 channel using riluzole, represents viable strategy to inhibit MB tumor growth by targeting potassium channels.
Supplementary Figure 4 EAG2 channel localizes to the trailing membrane during MB cell migration and regulates migratory polarity and cell motility
Immunofluorescence staining using EAG2 antibodies obtained from different commercial sources shows cell rear and trailing membrane enrichment during migration of multiple human MB cell lines, including SF8953-MB, DAOY, Vandy-MB-11, Vandy-MB-6 (at passage 3) and Vandy-MB-10 (at passage 3) cells. White arrows indicate migratory direction.
Supplementary Figure 5 KCNT2 channel does not enrich to the trailing edge during MB cell migration and does not regulate migratory polarity or cell motility
a, EAG2, but not KCNT2, displays cell rear and trailing edge enrichment during MB cell migration. b, KCNT2 knockdown does not considerably affect MB cell migratory polarization or the formation of lamellipodia (n = 482 and 391 randomly selected cells for Scr. shRNA and KCNT2 shRNA, respectively). c, 3-D reconstruction shows rear cell body contraction in control Vandy-MB-11 cells and cells with KCNT2 knockdown. d, Control Vandy-MB-11 cells and cells with KCNT2 knockdown show comparable migratory velocity (n = 20 for each group, two-tailed Student’s t-test). Each colored trace represents the migratory route of an individual Vandy-MB-11 cell over 5 hours. e, 5 μM thioridazine treatment for 3 days results in significantly reduced KCNT2 expression in Vandy-MB-11 cells (n = 4 independent cultures and qPCR experiments, two-tailed Student’s t-test). Error bars show ± SEM.
Supplementary Figure 6 Promazine blocks EAG2 channel and inhibits MB cell growth
a, Chemical structure of promazine. b, Promazine treatment markedly inhibits MB cell growth at 10 μM. c, Patch clamp recording shows that promazine significantly blocks EAG2 channel at 10 μM (n = 3, two-tailed Student’s t-test). Error bars show ± SEM.
Supplementary Figure 7 Working model: localized EAG2 potassium channel activity at the trailing edge regulates MB cell motility
EAG2 channel enriched at the trailing edge regulates local potassium efflux, which accompanies water loss to promote cell rear contraction and cell motility.
Supplementary Figure 8 Astemizole treatment of mice bearing subcutaneously allografted Math1-Cre; SmoM2 MB tumors
a, Astemizole treatment reduces MB cell clonal growth as neurospheres (Math1-Cre; SmoM2 cells) or monolayer colonies (Vandy-MB-11, DAOY and Ptch1+/-; p53-/- cells). b, Experimental design for acute allografting of Math1-Cre; SmoM2 MB tumors into the flanks of nu/nu BALB/C mice and astemizole treatment by oral gavage. Once palpable tumors are detected one-week post implantation, daily oral gavage of 50 mg/kg astemizole to the mice was performed for two weeks. c, 14 days of daily oral gavage of astemizole reduces proliferation and increases apoptosis in subcutaneously allografted Math1-Cre; SmoM2 tumors. d, Quantifications of tumor volumes show significantly inhibited tumor growth with astemizole treatment (n = 9 for vehicle and astemizole group, Two-Way ANOVA). Error bars show ± SEM.
Supplementary Figure 9 Full-length Western blotting images for cropped images shown in Fig. 6b
The full-length Western blotting images for the cropped images in Fig. 6b are shown with molecular weights indicated by protein ladder. The same membrane was first blotted with EAG2 antibody, followed by antibody stripping and re-blotting with α tubulin antibody. Chemiluminescence was imaged using Molecular Imager@VersaDoc MP4000 system (Bio-Rad).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1638 kb)
Rights and permissions
About this article
Cite this article
Huang, X., He, Y., Dubuc, A. et al. EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nat Neurosci 18, 1236–1246 (2015). https://doi.org/10.1038/nn.4088
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4088
This article is cited by
-
Targeting network circuitry in glioma
Nature Cancer (2023)
-
Pediatric brain tumors: a bibliometric analysis
Child's Nervous System (2022)
-
Elevated Kir2.1/nuclear N2ICD defines a highly malignant subtype of non-WNT/SHH medulloblastomas
Signal Transduction and Targeted Therapy (2022)
-
Drug Repurposing in Medulloblastoma: Challenges and Recommendations
Current Treatment Options in Oncology (2021)
-
Identification of undecylenic acid as EAG channel inhibitor using surface plasmon resonance-based screen of KCNH channels
BMC Pharmacology and Toxicology (2019)