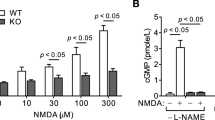
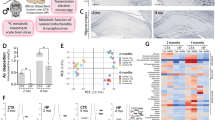
Abstract
Uptake of the neurotransmitter glutamate is effected primarily by transporters expressed on astrocytes, and downregulation of these transporters leads to seizures and neuronal death. Neurons also express a glutamate transporter, termed excitatory amino acid carrier–1 (EAAC1), but the physiological function of this transporter remains uncertain. Here we report that genetically EAAC1-null (Slc1a1−/−) mice have reduced neuronal glutathione levels and, with aging, develop brain atrophy and behavioral changes. EAAC1 can also rapidly transport cysteine, an obligate precursor for neuronal glutathione synthesis. Neurons in the hippocampal slices of EAAC1−/− mice were found to have reduced glutathione content, increased oxidant levels and increased susceptibility to oxidant injury. These changes were reversed by treating the EAAC1−/− mice with N-acetylcysteine, a membrane-permeable cysteine precursor. These findings suggest that EAAC1 is the primary route for neuronal cysteine uptake and that EAAC1 deficiency thereby leads to impaired neuronal glutathione metabolism, oxidative stress and age-dependent neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001).
Rothstein, J.D. et al. Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 (1994).
Shashidharan, P. et al. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 773, 139–148 (1997).
Arriza, J.L., Eliasof, S., Kavanaugh, M.P. & Amara, S.G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. USA 94, 4155–4160 (1997).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997).
Peghini, P., Janzen, J. & Stoffel, W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 16, 3822–3832 (1997).
Rothstein, J.D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Coco, S. et al. Non-synaptic localization of the glutamate transporter EAAC1 in cultured hippocampal neurons. Eur. J. Neurosci. 9, 1902–1910 (1997).
Sepkuty, J.P. et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J. Neurosci. 22, 6372–6379 (2002).
Zerangue, N. & Kavanaugh, M.P. Interaction of L-cysteine with a human excitatory amino acid transporter. J. Physiol. (Lond.) 493, 419–423 (1996).
Bendahan, A., Armon, A., Madani, N., Kavanaugh, M.P. & Kanner, B.I. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J. Biol. Chem. 275, 37436–37442 (2000).
Wu, G., Fang, Y.Z., Yang, S., Lupton, J.R. & Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 134, 489–492 (2004).
Dringen, R., Pfeiffer, B. & Hamprecht, B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 19, 562–569 (1999).
Shanker, G., Allen, J.W., Mutkus, L.A. & Aschner, M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 902, 156–163 (2001).
Murphy, T.H., Schnaar, R.L. & Coyle, J.T. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 4, 1624–1633 (1990).
Sagara, J.I., Miura, K. & Bannai, S. Maintenance of neuronal glutathione by glial cells. J. Neurochem. 61, 1672–1676 (1993).
Sato, H. et al. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J. Neurosci. 22, 8028–8033 (2002).
Baker, D.A. et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 6, 743–749 (2003).
Wang, X.F. & Cynader, M.S. Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 74, 1434–1442 (2000).
Dringen, R., Gutterer, J.M., Gros, C. & Hirrlinger, J. Aminopeptidase N mediates the utilization of the glutathione precursor CysGly by cultured neurons. J. Neurosci. Res. 66, 1003–1008 (2001).
Chen, Y. & Swanson, R.A. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J. Neurochem. 84, 1332–1339 (2003).
Himi, T., Ikeda, M., Yasuhara, T., Nishida, M. & Morita, I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J. Neural Transm. 110, 1337–1348 (2003).
Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62, 649–671 (2000).
Jain, A., Martensson, J., Stole, E., Auld, P.A. & Meister, A. Glutathione deficiency leads to mitochondrial damage in brain. Proc. Natl. Acad. Sci. USA 88, 1913–1917 (1991).
Schulz, J.B., Lindenau, J., Seyfried, J. & Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 267, 4904–4911 (2000).
Yang, Y., Kinney, G.A., Spain, W.J., Breitner, J.C. & Cook, D.G. Presenilin-1 and intracellular calcium stores regulate neuronal glutamate uptake. J. Neurochem. 88, 1361–1372 (2004).
Morris, R. Development of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984).
Hogg, N., Darley-Usmar, V.M., Wilson, M.T. & Moncada, S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem. J. 281, 419–424 (1992).
Himi, T., Ikeda, M., Yasuhara, T. & Murota, S.I. Oxidative neuronal death caused by glutamate uptake inhibition in cultured hippocampal neurons. J. Neurosci. Res. 71, 679–688 (2003).
Hogg, N., Singh, R.J. & Kalyanaraman, B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 382, 223–228 (1996).
Beckman, J.S. & Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271, C1424–C1437 (1996).
Griffith, O.W. & Meister, A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254, 7558–7560 (1979).
Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1, 145–157 (1997).
Avshalumov, M.V. & Rice, M.E. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J. Neurophysiol. 87, 2896–2903 (2002).
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A. & Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462–465 (1984).
Grover, L.M. & Yan, C. Blockade of GABAA receptors facilitates induction of NMDA receptor-independent long-term potentiation. J. Neurophysiol. 81, 2814–2822 (1999).
Mazor, D. et al. Red blood cell permeability to thiol compounds following oxidative stress. Eur. J. Haematol. 57, 241–246 (1996).
Parsons, J.L. & Chipman, J.K. The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis 15, 311–316 (2000).
Corcoran, G.B. & Wong, B.K. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 238, 54–61 (1986).
Umansky, V. et al. Glutathione is a factor of resistance of Jurkat leukemia cells to nitric oxide-mediated apoptosis. J. Cell. Biochem. 78, 578–587 (2000).
Zerangue, N. & Kavanaugh, M.P. Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 (1996).
Smith, C.P. et al. Assignment of the gene coding for the human high-affinity glutamate transporter EAAC1 to 9p24: potential role in dicarboxylic aminoaciduria and neurodegenerative disorders. Genomics 20, 335–336 (1994).
Gonzalez, M.I., Kazanietz, M.G. & Robinson, M.B. Regulation of the neuronal glutamate transporter excitatory amino acid carrier-1 (EAAC1) by different protein kinase C subtypes. Mol. Pharmacol. 62, 901–910 (2002).
Canolle, B. et al. Glial soluble factors regulate the activity and expression of the neuronal glutamate transporter EAAC1: implication of cholesterol. J. Neurochem. 88, 1521–1532 (2004).
Aoyama, K. et al. Acidosis causes endoplasmic reticulum stress and caspase-12-mediated astrocyte death. J. Cereb. Blood Flow Metab. 25, 258–370 (2005).
Suh, S.W. et al. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J. Neurosci. 23, 10681–10690 (2003).
Hochman, D.W. & Schwartzkroin, P.A. Chloride-cotransport blockade desynchronizes neuronal discharge in the “epileptic” hippocampal slice. J. Neurophysiol. 83, 406–417 (2000).
Yin, H.Z., Sensi, S.L., Ogoshi, F. & Weiss, J.H. Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci. 22, 1273–1279 (2002).
Meister, A., Anderson, M.E. & Hwang, O. Intracellular cysteine and glutathione delivery systems. J. Am. Coll. Nutr. 5, 137–151 (1986).
Lantz, R.C., Lemus, R., Lange, R.W. & Karol, M.H. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol. Sci. 60, 348–355 (2001).
Acknowledgements
We thank D. Burns for technical assistance and M. Yenari and S. Massa for critical reading of the manuscript. This work was supported by grants from the US National Institutes of Health and the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Markers of oxidant stress are absent from corpus callosum of aged EAAC1−/− mice. (PDF 416 kb)
Rights and permissions
About this article
Cite this article
Aoyama, K., Suh, S., Hamby, A. et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 9, 119–126 (2006). https://doi.org/10.1038/nn1609
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1609
This article is cited by
-
Developmental impact of glutamate transporter overexpression on dopaminergic neuron activity and stereotypic behavior
Molecular Psychiatry (2022)
-
Naringin Confers Protection against Psychosocial Defeat Stress-Induced Neurobehavioral Deficits in Mice: Involvement of Glutamic Acid Decarboxylase Isoform-67, Oxido-Nitrergic Stress, and Neuroinflammatory Mechanisms
Journal of Molecular Neuroscience (2021)
-
Early-life stress induces EAAC1 expression reduction and attention-deficit and depressive behaviors in adolescent rats
Cell Death Discovery (2020)
-
Amphetamine Stimulates Endocytosis of the Norepinephrine and Neuronal Glutamate Transporters in Cultured Locus Coeruleus Neurons
Neurochemical Research (2020)
-
Differential effects of N-acetylcysteine on retinal degeneration in two mouse models of normal tension glaucoma
Cell Death & Disease (2019)