Abstract
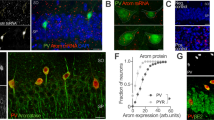
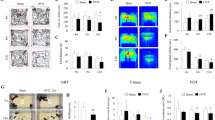
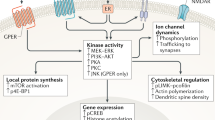
Estrogens have long been implicated in influencing cognitive processes, yet the molecular mechanisms underlying these effects and the roles of the estrogen receptors alpha (ERα) and beta (ERβ) remain unclear. Using pharmacological, biochemical and behavioral techniques, we demonstrate that the effects of estrogen on hippocampal synaptic plasticity and memory are mediated through ERβ. Selective ERβ agonists increased key synaptic proteins in vivo, including PSD-95, synaptophysin and the AMPA-receptor subunit GluR1. These effects were absent in ERβ knockout mice. In hippocampal slices, ERβ activation enhanced long-term potentiation, an effect that was absent in slices from ERβ knockout mice. ERβ activation induced morphological changes in hippocampal neurons in vivo, including increased dendritic branching and increased density of mushroom-type spines. An ERβ agonist, but not an ERα agonist, also improved performance in hippocampus-dependent memory tasks. Our data suggest that activation of ERβ can regulate hippocampal synaptic plasticity and improve hippocampus-dependent cognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
McEwen, B. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 57, 357–384 (2002).
Pfaff, D.W. et al. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women's health. J. Steroid Biochem. Mol. Biol. 74, 365–373 (2000).
Gruber, C.J., Tschugguel, W., Schneeberger, C. & Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 346, 340–352 (2002).
Rissman, E.F., Wersinger, S.R., Taylor, J.A. & Lubahn, D.B. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm. Behav. 31, 232–243 (1997).
Krege, J.H. et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 95, 15677–15682 (1998).
Shughrue, P.J., Lane, M.V. & Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525 (1997).
Bliss, T.V. & Collingridge, G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Mitra, S.W. et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144, 2055–2067 (2003).
Herrick, S.P., Waters, E.M., Drake, C.T., McEwen, B.S. & Milner, T.A. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 1121, 46–58 (2006).
Milner, T.A. et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 491, 81–95 (2005).
Yaffe, K. et al. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet 356, 708–712 (2000).
Maki, P.M., Rich, J.B. & Rosenbaum, R.S. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia 40, 518–529 (2002).
Savonenko, A.V. & Markowska, A.L. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 119, 821–830 (2003).
Frye, C.A. & Rhodes, M.E. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 956, 285–293 (2002).
Sandstrom, N.J. & Williams, C.L. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 115, 384–393 (2001).
Woolley, C.S. & McEwen, B.S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 12, 2549–2554 (1992).
Li, C. et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. USA 101, 2185–2190 (2004).
Adams, M.M., Fink, S.E., Janssen, W.G., Shah, R.A. & Morrison, J.H. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J. Comp. Neurol. 474, 419–426 (2004).
Woolley, C.S., Weiland, N.G., McEwen, B.S. & Schwartzkroin, P.A. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor–mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 17, 1848–1859 (1997).
Smith, C.C. & McMahon, L.L. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J. Neurosci. 26, 8517–8522 (2006).
Zhou, Y., Watters, J.J. & Dorsa, D.M. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137, 2163–2166 (1996).
Rissman, E.F., Heck, A.L., Leonard, J.E., Shupnik, M.A. & Gustafsson, J.A. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. USA 99, 3996–4001 (2002).
Day, M., Sung, A., Logue, S., Bowlby, M. & Arias, R. Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav. Brain Res. 164, 128–131 (2005).
Rhodes, M.E. & Frye, C.A. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol. Learn. Mem. 85, 183–191 (2006).
Rudick, C.N. & Woolley, C.S. Selective estrogen receptor modulators regulate phasic activation of hippocampal CA1 pyramidal cells by estrogen. Endocrinology 144, 179–187 (2003).
Malinow, R. & Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Beique, J.C. et al. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. USA 103, 19535–19540 (2006).
Ehrlich, I., Klein, M., Rumpel, S. & Malinow, R. PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. USA 104, 4176–4181 (2007).
Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Shi, S., Hayashi, Y., Esteban, J.A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Man, H.Y., Sekine-Aizawa, Y. & Huganir, R.L. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. USA 104, 3579–3584 (2007).
Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 26, 360–368 (2003).
Moriarty, K., Kim, K.H. & Bender, J.R. Minireview: estrogen receptor–mediated rapid signaling. Endocrinology 147, 5557–5563 (2006).
Barco, A., Pittenger, C. & Kandel, E.R. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin. Ther. Targets 7, 101–114 (2003).
Lee, S.J. et al. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience 124, 549–560 (2004).
Boulware, M.I. et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element–binding protein. J. Neurosci. 25, 5066–5078 (2005).
Zamanillo, D. et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811 (1999).
Reisel, D. et al. Spatial memory dissociations in mice lacking GluR1. Nat. Neurosci. 5, 868–873 (2002).
Gazzaley, A.H., Weiland, N.G., McEwen, B.S. & Morrison, J.H. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 16, 6830–6838 (1996).
Cyr, M., Thibault, C., Morissette, M., Landry, M. & Di Paolo, T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology 25, 242–257 (2001).
Kopec, C.D., Real, E., Kessels, H.W. & Malinow, R. GluR1 links structural and functional plasticity at excitatory synapses. J. Neurosci. 27, 13706–13718 (2007).
MacLennan, A.H. et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause 13, 28–36 (2006).
Morrison, J.H. & Hof, P.R. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer's disease. Prog. Brain Res. 136, 467–486 (2002).
Ostlund, H., Keller, E. & Hurd, Y.L. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. NY Acad. Sci. 1007, 54–63 (2003).
Kuramoto, N. et al. Phospho-dependent functional modulation of GABAB receptors by the metabolic sensor AMP-dependent protein kinase. Neuron 53, 233–247 (2007).
Wang, S.X., Ikeda, M. & Guggino, W.B. The cytoplasmic tail of large conductance, voltage- and Ca2.-activated K. (MaxiK) channel is necessary for its cell surface expression. J. Biol. Chem. 278, 2713–2722 (2003).
Roy, E.J. & McEwen, B.S. An exchange assay for estrogen receptors in cell nuclei of the adult rat brain. Steroids 30, 657–669 (1977).
Lein, P.J. et al. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ. Health Perspect. 115, 556–563 (2007).
Neigh, G.N. et al. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in CA1 pyramidal cells and selectively alters acquisition of spatial memory. Eur. J. Neurosci. 20, 1865–1872 (2004).
Harris, H.A. et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology 144, 4241–4249 (2003).
Acknowledgements
We thank T. Comery, M. Hayoun and M. Sharma for their technical assistance. We are also grateful to A. Randall, E. Trybulski and H. Harris for their critical comments on this manuscript. S.J.M. is supported by US National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS 046478, 048045, 051195 056359, P01NS054900 and the UK Medical Research Council.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 204 kb)
Rights and permissions
About this article
Cite this article
Liu, F., Day, M., Muñiz, L. et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11, 334–343 (2008). https://doi.org/10.1038/nn2057
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2057
This article is cited by
-
Sex and interspecies differences in ESR2-expressing cell distributions in mouse and rat brains
Biology of Sex Differences (2023)
-
Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders
Pharmacological Reports (2023)
-
Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus
Nature Communications (2022)
-
Transcriptomic Profile Identifies Hippocampal Sgk1 as the Key Mediator of Ovarian Estrogenic Regulation on Spatial Learning and Memory and Aβ Accumulation
Neurochemical Research (2022)
-
Effects of oral contraceptive pills on mood and magnetic resonance imaging measures of prefrontal cortical thickness
Molecular Psychiatry (2021)