Abstract
Accumulating evidence suggests that the pathophysiology of depression might be associated with neuroinflammation, which could be attenuated by pharmacological treatment for depression. Omega-3 polyunsaturated fatty acids (PUFAs) are anti-inflammatory and exert antidepressant effects. The aim of this study was to identify the molecular mechanisms through which docosahexaenoic acid (DHA), the main omega-3 PUFA in the brain, modulates oxidative reactions and inflammatory cytokine production in microglial and neuronal cells. The results of this study showed that DHA reduced expressions of tumor necrosis factor-α, interleukin-6, nitric oxide synthase, and cyclo-oxygenase-2, induced by interferon-γ, and induced upregulation of heme oxygenase-1 (HO-1) in BV-2 microglia. The inhibitory effect of DHA on nitric oxide production was abolished by HO-1 inhibitor zinc protoporphyrin IX. In addition, DHA caused AKT and ERK activation in a time-dependent manner, and the DHA-induced HO-1 upregulation could be attenuated by PI-3 kinase/AKT and MEK/ERK inhibitors. DHA also increased IKKα/β phosphorylation, IκBα phosphorylation, and IκBα degradation, whereas both nuclear factor-κB and IκB protease inhibitors could inhibit DHA-induced HO-1 expressions. The other major n-3 PUFA, eicosapentaenoic acid, showed similar effects of DHA on inflammation and HO-1 in repeated key experiments. In connecting with inflammation hypothesis of depression and clinical studies supporting the antidepressant effects of omega-3 PUFAs, this study provides a novel implication of the antidepressant mechanisms of DHA.
Similar content being viewed by others
INTRODUCTION
There is compelling evidence to suggest that neuroinflammation and inflammatory mediator production contribute to the pathophysiology of depression. For example, depressed patients have been found to have higher levels of proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), as well as interferon-γ (IFN-γ; Maes et al, 2009; Maes, 2008). From prospective observations of animal and clinical studies, behavioral changes induced by proinflammatory cytokines, eg, IL-1β, IFN-γ, and IFN-α, resemble symptoms of depression, and are probably the most strikingly supportive evidence for the inflammation theory of depression (Dantzer et al, 2008; Miller et al, 2009; Su, 2009). In fact, inflammatory cytokines have been found to interact with many pathophysiological domains, including neurotransmitter metabolisms, neuroendocrine functions, synaptic plasticity, and oxidative status, which might characterize depression (Raison et al, 2006). The neuro-inflammatory activation contributes to the overproduction of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) and the loss of glial elements (Ida et al, 2008; Li et al, 2008b, 1999), which are consistent with neuropathological findings characterizing depression (Miller et al, 2009). Interestingly, antidepressant medications, including noradrenaline reuptake inhibitors (O’Sullivan et al, 2009) and tricyclic antidepressants (TCAs; Tai et al, 2006), have been found to attenuate the oxidative stress and inflammatory reaction by suppressing the expression of iNOS and several proinflammatory cytokines.
The mechanism of antioxidative and anti-inflammatory reactions from antidepressant medications has been found to be associated with an enhancement of heme oxygenase-1 (HO-1) expression (Kim et al, 2008; Shin et al, 2009; Tai et al, 2009). HO is a rate-limiting enzyme that catalyzes the conversion of heme to equimolar amounts of CO, iron, and biliverdin (Abraham and Kappas, 2008). Two main HO isoforms, HO-1 and HO-2, can be found in human brain. Unlike the constitutively expressed HO-2, HO-1 (also referred to as heat-shock protein 32, HSP32) can be strongly inducible in response to diverse stress-related cellular stimuli, including its substrate heme, heavy metals, UV radiation, endotoxin, inflammatory cytokines, and prostaglandins (Abraham and Kappas, 2008). The induction of HO-1 in brain neuronal and nonneuronal cells is important in neuroprotection and neuroplasticity (Choi and Kim, 2008; Le et al, 1999), which are also characteristics of antidepressant mechanisms (Charney and Manji, 2004; Krishnan and Nestler, 2008).
N-3 (or omega-3) polyunsaturated fatty acids (PUFAs) have an important role in neurobiological mechanisms of depression (Horrobin and Bennett, 1999; Su et al, 2000) and cytokine-induced sickness and depressive behaviors (Song et al, 2004a; Su et al, 2010; Su, 2008). Specifically, societies consuming a large amount of omega-3 PUFAs seem to have a lower prevalence of major depressive disorder (Hibbeln, 1998). In addition, major depression is associated with low levels of n-3 PUFAs in peripheral blood tissues (Lin et al, 2010; Maes et al, 1996; Peet et al, 1998) and in postmortem brains (McNamara et al, 2007). Consistent with the observational studies, several clinical trials, if not all (Marangell et al, 2003; Silvers et al, 2005), have shown that omega-3 PUFAs were effective in treating patients with major depression. (Freeman et al, 2006; Lin and Su, 2007; Su et al, 2008). Moreover, n-3 PUFAs have a protective effect against the development of depression-like behaviors induced by stress (Carlezon et al, 2005; Huang et al, 2008) or proinflammatory cytokines (Song et al, 2004a, 2007, 2008) in animal models of depression.
The function of n-3 PUFAs in the brain is diverse, but the mechanisms of antidepressant effects of n-3 PUFAs have yet to be elucidated. Although n-3 PUFAs can regulate monoamine neurotransmitters that interact with central pathophysiological domains in depression, accumulating evidence implies that anti-inflammation might also be involved (Su, 2009; Venna et al, 2009). The two major bioactive components of n-3 PUFAs, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), particularly found in fatty fish, are not synthesized in the human body and should be obtained directly from the diet (Lands, 1992). EPA has an important role in balancing inflammation by antagonizing membrane arachidonic acid (an n-6 PUFA), reducing prostaglandin E2 synthesis (Farooqui et al, 2006), and reversing inflammatory responses induced by cytokines and LPS in microglia (Kawashima et al, 2009; Lynch et al, 2007; Moon and Pestka, 2003), and has also been found to have beneficial effects on cytokine-induced sickness and depressive behaviors (Song et al, 2004a, 2004b, 2008), as well as antidepressant effects in patients with depression (Nemets et al, 2006; Peet and Horrobin, 2002; Su et al, 2003). Compared with only 0.1% of EPA in total brain fatty acid, DHA comprises 10–20% and is the main n-3 fatty acid in the brain (McNamara and Carlson, 2006) and has long been considered neuroprotective (Bazan, 2006) and anti-inflammatory (De Smedt-Peyrusse et al, 2008; Ebert et al, 2009). However, the antidepressant mechanisms through neuroinflammation pathways for DHA still remain unclear.
Recently, Brand et al (2010) have found that membrane lipid modification by PUFAs, especially DHA, promoted upregulation of HO-1, but sensitized oligodendroglial cells to oxidative stress. Their study, however, applied stimuli from heat and H2O2 oxidative stresses, rather than inflammatory stimuli. To our knowledge, the role of HO-1 in anti-inflammatory effects of DHA has not yet been reported. In this study, we applied an in vitro cell model to show DHA's effects on inflammatory stimulation in immortalized microglial cells, BV-2. Microglial cells reside in the parenchyma and have a critical role in host defense, tissue repair, and neuroprotection/neurotoxicity in the brain (Hanisch and Kettenmann, 2007). Microglial activation involves changes in cell phenotype and the expression of new proteins such as iNOS and cyclo-oxygenase-2 (COX-2), which in turn might result in neuronal damages through the release of nitric oxide (NO) and proinflammatory cytokines (Bal-Price and Brown, 2001; Block et al, 2007). Active microglial cells can be immortalized into the BV-2 cell line, which retains most of the morphological, phenotypical, and functional properties of freshly isolated microglial cells (Blasi et al, 1990). The goal of this study was to identify the molecular mechanism through which DHA modulates oxidative reaction and proinflammatory cytokine production in microglial cells.
MATERIALS AND METHODS
Materials
DHA and EPA were purchased from Sigma-Aldrich (St Louis, MO). Fetal bovine serum (FBS), DMEM, and OPTI-MEM were purchased from Gibco BRL (Invitrogen Life Technologies, Carlsbad, CA). Goat anti-mouse and anti-rabbit horseradish peroxidase-conjugated IgG, primary antibodies against IκBα, IKKα/β, p65, p50, PI-3 kinase (p85), β-actin, AKT, ERK2, phospho-ERK1/2, and phospho-AKT (Ser473), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies against IKKα/β, phosphorylated at Ser180/181, and p65, phosphorylated at Ser573, were purchased from Cell Signaling and Neuroscience (Danvers, MA). Wortmannin, TPCK, and PDTC were purchased from Calbiochem (San Diego, CA). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO).
Cell Culture
All protocols for animal experiments were approved by Animal Care Committees of China Medical University. Sprague–Dawley rats were obtained from the National Laboratory Animal Center in Taiwan. The detailed procedures of primary microglia cultures have been described elsewhere (Lu et al, 2009, 2007). Briefly, glial cells were cultured for 12–14 days in DMEM/F12 (Gibco, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. To separate microglia, flasks were shaken for 5 h at 180 r.p.m. in a rotary shaker at 37°C. Detached cells were passed through a 70 μm nylon mesh filter (BD Falcon, Franklin Lakes, NJ) and then plated into 24-well plates at a density of 5 × 105 cells/well. The purity of microglia cultures was assessed using CD11b antibody, and more than 95% of cells were stained positively. Cells were cultured in DMEM with 2% FBS for 2 days before drug treatment.
BV-2, the murine cell line, has been generated by infecting primary microglial cell cultures with a v-raf/v-myc oncogene carrying retrovirus (J2). Phenotypically, BV-2 cells tested positive for MAC1 and MAC2 antigens. As BV-2 cells retain most of the morphological, phenotypical, and functional properties described for freshly isolated microglial cells, they can be considered as immortalized active microglial cells (Blasi et al, 1990). Cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) with 10% FBS at 37°C in a humidified incubator in an atmosphere of 5% CO2 and 95% air. Confluent cultures were passaged by trypsinization.
MES23.5 is a dopaminergic cell line hybridized from murine neuroblastoma-glioma N18TG2 cells with rat mesencephalic neurons, which show several properties similar to those of primary neurons originated in the substantia nigra (Crawford et al, 1992). Cells were cultured in DMEM/F12 containing Sato's components growth medium supplemented with 5% FBS, 100 U/ml of penicillin, and 100 mg/ml of streptomycin at 37°C, in a humid 5% CO2, 95% air environment. For experiments, cells were seeded at a density of 1 × 105/cm2 in plastic plates.
Western Blot Analysis
BV-2 cells were treated with DHA for indicated time periods and then washed with cold PBS that had been lysed for 30 min on ice with radioimmunoprecipitation assay buffer (50 mM HEPES (PH 7.4), 150 mM NaCl, 4 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% Triton X-100, 0.25% sodium deoxycholate, 50 mM 4-(2-aminoethyl) benzene sulfonylfluoride, 50 μg/ml leupeptin, and 20 μg/ml aprotinin). Protein samples were separated using sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% nonfat milk in PBS for 1 h at room temperature and then probed with primary antibodies. After undergoing three PBS washes, the membranes were incubated with secondary antibodies. Blots were visualized by enhanced chemiluminescence using a Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY).
Reverse Transcription and Quantitative Real-Time PCR
Total RNA was extracted from BV-2 cells using a TRIzol kit (MDBio, Taipei, Taiwan). The reverse transcription reaction was performed using 2 μg of total RNA that was reverse transcribed into cDNA.
Quantitative real-time PCR was performed using SYBR-green detection of PCR products in real time using the 48-well StepOne Real-Time System (Applied Biosystems, Foster City, CA). The following sequence-specific oligonucleotide primers were used:
iNOS: 5′-CCCAGAGTTCCAGCTTCTGG-3′ and
5′-CCAAGCCCCTCACCATTATCT-3′;
COX-2: 5′-TGGGGTGATGAGCAACTATT-3′ and 5′-AAGGAGCTCTGGGTCAAACT-3′;
IL-1β: 5′-TGGGGGAGATTCTCACTTTG-3′ and 5′-CCATCAGCGTTCCCATACTT-3′;
IL-6: 5′-CCAGTTGCCTTCTTGGGACTG-3′ and
5′-CAGGTCTGTTGGGAGTGGTATCC-3′;
TNF-α: 5′-AAAATTCGAGTGACAAGCCTGTAG-3′ and
5′-CCCTTGAAGAGAACCTGGGAGTAG-3′;
GAPDH: 5′-CTCAACTACATGGTCTACATGTTCCA-3′ and 5′-CTTCCCATTCTCAGCCTTGACT-3′.
Immunocytofluorescent Staining
Cells were seeded onto glass coverslips and exposed to DHA for 24 h, then washed with PBS and fixed with 4% paraformaldehyde for 15 min, after which they were permeabilized with Triton X-100 for 30 min. After blocking with 5% nonfat milk in PBS buffer, cells were incubated with rabbit anti-p50 or anti-p65 antibodies for 1 h at room temperature. After a brief wash, cells were incubated with a goat anti-rabbit FITC-conjugated secondary antibody (1 : 100; Leinco Tec., St Louis, MO). Finally, the cells were washed again, mounted, and visualized with a fluorescence microscope.
Statistics
The values given are means±SEM. The significance of difference between the experimental and control groups was assessed by Student's t-test. The difference was significant if the p-value was <0.05.
RESULTS
DHA Suppresses IFN-γ-Induced Inflammatory Cytokine Expression in Bv-2 Microglia
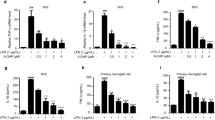
We used the BV-2 microglia cell line to study the antineuroinflammatory mechanism of DHA. To determine the effect of DHA on iNOS and COX-2 protein levels, BV-2 cells were treated with LPS or IFN-γ plus DHA, and protein levels were detected using western blotting (Figure 1a and b). BV-2 cells were pretreated with various concentrations of DHA for 30 min and then stimulated with IFN-γ for another 24 h. As shown in Figure 1c and d, DHA effectively inhibited IFN-γ-induced iNOS and COX-2 expression in a concentration-dependent manner. The cell culture medium was then harvested to determine nitrite content by Griess reaction. Results show that IFN-γ increased the production of NO up to 5.5-fold. DHA effectively antagonized IFN-γ-induced NO production (Figure 1e). To determine whether the mechanisms are similar across n-3 PUFAs, we repeated the experiments with EPA and found that EPA inhibited IFN-γ-induced iNOS and COX-2 expression (Supplementary Figure S1A), as well as NO production (Supplementary Figure S1B), in BV-2 microglia. DHA and EPA at concentrations ranging from 3 to 30 μM did not affect cell viability using the MTT assay (data not shown).
DHA suppresses IFN-γ-induced iNOS and COX-2 expression. BV-2 cells were pretreated with DHA (30 μM) for 30 min before application of LPS (100 ng/ml) or IFN-γ (30 μg/ml). Whole-cell lysis protein was extracted and subjected to western blot analysis for iNOS (a) and COX-2 (b) expression after 24 h of incubation with LPS or IFN-γ. Cells were pretreated with various concentrations of DHA (0, 3, 10, or 30 μM) for 30 min before IFN-γ (30 μg/ml) application. Whole-cell lysis protein was extracted and subjected to western blot analysis for iNOS (c) and COX-2 (d) expression after 24 h of incubation with IFN-γ. (e) The culture mediums were collected and analyzed using Griess reaction. (f) BV-2 cells were pretreated with various concentrations of DHA (0, 3, 10, or 30 μM) for 30 min before being stimulated with IFN-γ (30 μg/ml) for 6 h. Relative mRNA levels of iNOS, COX-2, TNF-α, and IL-6 were analyzed using real-time PCR and normalized with GAPDH. Each bar represents means±SEM. *P<0.05 compared with the control group. #P<0.05 compared with the IFN-γ treatment group. Similar results were obtained from at least four independent experiments.
With real-time PCR analysis, we examined the effects of DHA and IFN-γ on BV-2 microglial expressions in mRNA levels of iNOS, COX-2, IL-6, and TNF-α. Not shown in the figures, IFN-γ induced iNOS, COX-2, IL-6, and TNF-α expressions in a time- and concentration-dependent manner. We then analyzed levels of total mRNA extracted from BV-2 microglia after a 6-h IFN-γ stimulation. As shown in Figure 1f, pretreatment with DHA significantly inhibited IFN-γ-induced iNOS, COX-2, IL-6, and TNF-α mRNA expressions.
DHA Induces HO-1 Upregulation in BV-2 Microglia Cells
As HO-1 induction participates in the negative regulation of inflammatory cytokine expression, we investigated whether DHA leads to HO-1 induction. When BV-2 microglia were treated with DHA for 24 h, HO-1 levels were increased in a concentration-dependent manner (Figure 2a). Similarly, EPA also increased HO-1 protein expression in BV-2 microglia (Supplementary Figure S2A). In Figure 2b and c, we further analyzed HO-1 mRNA expressions induced by DHA in various concentration- and time-dependent conditions in BV-2. We also analyzed HO-1 mRNA expressions induced by DHA (Figure 2d) and EPA (Supplementary Figure S2B) in primary cultured microglia. We then used ZnPPIX, an HO-1 inhibitor, to examine whether HO-1 expression is required in the anti-inflammatory pathways exerted by DHA. As shown in Figure 2d, the presence of ZnPPIX abrogated the inhibitory effects of DHA on IFN-γ-induced NO production. Similarly, the inhibitory effects of EPA on IFN-γ-induced NO production could also be abrogated by ZnPPIX treatment (Supplementary Figure S2C).
DHA induces HO-1 upregulation in BV-2 microglia cells. (a) BV-2 cells were stimulated with various concentrations of DHA for 24 h. Whole-cell lysates were subjected to western blot analysis using an antibody against HO-1. Cells were stimulated with various concentrations of DHA for 6 h (b) or stimulated with DHA (30 μM) for indicated time periods (c). Rat primary cultured microglia were stimulated with DHA (30 μM) for 6 h (d). The mRNA level of HO-1 was analyzed using real-time PCR. Results are expressed as the mean±SEM of four independent experiments. (e) Cells were pretreated with ZnPPIX (10 μM) for 30 min and treated with DHA for another 30 min before IFN-γ (30 μg/ml) application. NO production was analyzed using Griess reaction. Note that ZnPPIX effectively antagonized IFN-γ-induced NO production. Each bar represents means±SEM from at least four independent experiments. *p<0.05 compared with the control group; #p<0.05 compared with the IFN-γ treatment group.
DHA Stimulates HO-1 Upregulation Through PI-3 Kinase/AKT And ERK Signaling Pathways in BV-2 Microglia
We investigated the signaling pathway involved in the HO-1 expression induced by DHA. As shown in Figure 3a, DHA-induced HO-1 expression was markedly antagonized by pretreatment with the PI-3 kinase/AKT inhibitor LY294002 or the MEK inhibitor PD98059. These inhibitors did not affect cell viability as assessed using MTT assay (data not shown). Furthermore, DHA activated AKT and ERK1/2 phosphorylation in a time-dependent manner (Figure 3b and c). These data suggest that activation of PI-3 kinase/AKT and ERK pathways is required for the upregulation of HO-1 induced by DHA in BV-2 microglia.
DHA stimulates HO-1 upregulation through PI-3 kinase/AKT and ERK signaling pathways in BV-2 microglia. (a) BV-2 cells were pretreated with the PI-3 kinase inhibitor LY294002 (10 μM) or MEK inhibitor PD98059 (30 μM) for 30 min before DHA treatment. Whole-cell lysates were subjected to western blot analysis using an antibody against HO-1. (b and c) Cells were incubated with DHA for indicated time periods (0–120 min). Whole-cell lysates were subjected to western blot analysis using antibodies against the phosphorylation of AKT and ERK1/2. Similar results were obtained from at least three independent experiments.
DHA Induces HO-1 Expression Through The NF-κB Signaling Pathway
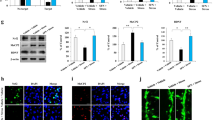
It has been reported that nuclear factor-κB (NF-κB) activation is important for HO-1 induction in many cells (Chen et al, 2004; Hill-Kapturczak et al, 2001; Juan et al, 2005; Lin et al, 2007; Nakao et al, 2008). We therefore examined whether the NF-κB pathway is involved in DHA-induced HO-1 expression. NF-κB activation was assessed with the translocation of NF-κB from cytosol to nucleus. As shown in Figure 4a, DHA treatment resulted in a marked translocation of NF-κB p50 and p65 from the cytosol to the nucleus in microglia. Furthermore, NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC, 30 μM) and IκBα phosphorylation inhibitor (Bay 117082, 3 μM) effectively antagonized the enhancement of HO-1 expression induced by DHA (Figure 4b). Again, treatment with those inhibitors did not affect cell viability assessed using MTT assay (data not shown). The immunocytochemical examination of NF-κB localization has also been examined. As shown in Figure 4c, NF-κB p50 and p65 were primarily located in the cytosol during the resting state (control panel), and were translocated into nuclei in response to DHA treatment for 1 h.
DHA induces HO-1 expression through the NF-κB signaling pathway. (a) BV-2 cells were treated with DHA for indicated time intervals, and the levels of cytosolic and nuclear p50 or p65 were determined by immunoblotting with a p65- or p50-specific antibody. PCNA and β-actin were used as loading controls for nuclear and cytosolic fractions, respectively. (b) Cells were pretreated with PDTC (30 μM) or Bay 11-7082 (3 μM) for 30 min, followed by stimulation for 24 h with DHA (30 μg/ml). Whole-cell lysis protein was subjected to western blot analysis for HO-1. (c) The nuclear translocation of p50 or p65 after 1 h of DHA treatment is shown using immunofluorescence assay. Scale bar, 10 μm.
We then examined the upstream molecules involved in DHA-induced NF-κB activation. Stimulation of cells with DHA induced IKKα/β phosphorylation, IκBα phosphorylation, and IκBα degradation in a time-dependent manner (Figure 5a and b). Pretreatment of cells with PI-3 kinase inhibitors (LY294002 or wortmannin) or MEK inhibitors (PD98059 or U0126) attenuated DHA-induced IKKα/β phosphorylation and IκBα phosphorylation (Figure 5d and e), suggesting that IKKα/β activation is regulated by PI-3 kinase/AKT or ERK signaling pathways. Treatment of BV-2 microglia cells with DHA also increased p65 phosphorylation at Ser573 in a time-dependent manner (Figure 5c). These results showed that DHA effectively inhibited IFN-γ-induced inflammatory mediator expression. In addition, DHA also induced HO-1 upregulation through the activation of PI-3 kinase/AKT and ERK signaling pathways, leading to IKKα/β phosphorylation, p65 phosphorylation, and activation of NF-κB in the microglia.
DHA induces IKKα/β activation, IκBα phosphorylation, IκBα degradation, and p65 Ser573 phosphorylation in BV-2 microglia. BV-2 cells were incubated with DHA for indicated time periods, and phospho-IKKα/β (a), phospho- IκBα and IκBα (b), or phosphor-p65 (c) expression levels were determined using western blot analysis. Note that DHA induced IKKα/β phosphorylation, IκBα phosphorylation and IκBα degradation, and p65 phosphorylation in a time-dependent manner. Cells were pretreated with LY294002 (10 μM) or wortmannin (100 nM) (d), or with PD98059 (30 μM) or U0126 (1 μM) (e), for 30 min, followed by stimulation with DHA for 60 min. Cell lysates were then evaluated using immunoblotting. Results are representative of four independent experiments.
DISCUSSION
The main finding of this study is that DHA potently inhibits oxidative reactions and proinflammatory responses in microglia, probably by promoting the upregulation of HO-1 expression. To our knowledge, this is the first report that shows the role of HO-1 in the anti-inflammatory effects of DHA and identifies the molecular mechanism through which DHA modulates oxidative reaction and proinflammatory cytokine production in microglial cells.
First, the inflammatory stimuli of IFN-γ increase the upregulation of oxidative and proinflammatory mediators, including iNOS, COX-2, IL-6, and TNF-α, which can be attenuated by DHA. The results of our current study are consistent with the findings from previous studies showing that DHA is antioxidative and anti-inflammatory (De Smedt-Peyrusse et al, 2008; Ebert et al, 2009; Komatsu et al, 2003; Saw et al, 2010). However, there are some studies showing that DHA is pro-oxidative in higher doses (Brand et al, 2010; Hirafuji et al, 2002). In the nervous system, DHA has diverse functions. For example, DHA can regulate membrane receptors and influence membrane fluidity at the molecular level, and promote neurite growth and neuronal survival at the cellular level (Su, 2009). Although antioxidant and anti-inflammatory effects of DHA may be prominent, its pro-oxidant potential and risk of an increase in lipid peroxide formation have to be taken into consideration (Brand et al, 2010; Yavin et al, 2002). Future studies investigating the paradoxical oxidant effects of DHA are warranted.
Second, our results support previous reports (Tsoyi et al, 2008; Vareille et al, 2008) showing that HO-1 is a critical regulator of NO production in numerous cells. Specifically, we found that DHA inhibition of NO production was effectively abolished by ZnPPIX, an HO-1 inhibitor. In addition, previous reports have shown that PI-3 kinase/AKT and ERK pathways regulate HO-1 expression in various cells (Choi and Kim, 2008; Wu et al, 2006). Again, our results from the primary cultured microglia and BV-2 microglial cells support the role of these pathways in DHA-induced HO-1 expression. Specifically, DHA activated AKT and ERK1/2 phosphorylation, and blockade of PI-3 kinase/AKT and MEK/ERK pathways inhibited DHA-induced HO-1 expression. Furthermore, the HO-1 upregulation induced by both DHA and EPA could be observed in MES 23.5 dopaminergic cells (Supplementary Figure S3). Further experiments would be needed to specify the effects of HO-1 expression in neuronal cells.
Interestingly, the activation of AKT, ERK1/2 phosphorylation, and NF-κB seems to be associated with mechanisms of antidepressant effects of several traditional antidepressants (eg, specific serotonin reuptake inhibitors and TCAs; Charney and Manji, 2004; Duman et al, 1999, 2000; Li et al, 2008a; Manji et al, 2001; Mercier et al, 2004) and off-label agents used for depression treatment (eg, lithium, valproate, omega-3 PUFAs, and atypical antipsychotics; Chen et al, 1999; Lu et al, 2004; Lu and Dwyer, 2005; Manji et al, 2001; Seti et al, 2009; Zhang et al, 2009). A recent report has shown that a TCA, amitriptyline, induces HO-1 expression in chronic morphine-infused rats (Tai et al, 2009). Interestingly, our additional experiment also showed that another TCA, desipramine, could increase HO-1 expression in BV-2 microglia. Furthermore, a desipramine cotreatment with DHA potentiates this HO-1 expression (Supplementary Figure S4, not shown in the Results section). In the regulatory level, the HO-1 gene promoter contains multiple potential regulatory transcription factor binding sites, including NF-κB, ARE, AP-1, and IL-6-responsive elements (Choi and Alam, 1996; Lavrovsky et al, 1994), suggesting a potential role for these transcription factors in modulating HO-1 expression. The NF-κB signaling pathway regulates several physiological processes and has been found to have a dual function in response to microenvironmental stimuli (Pasparakis, 2009). For example, NF-κB promotes the expression of proinflammatory genes, including genes encoding cytokines, chemokines, and adhesion molecules (Lu et al, 2009, 2007). On the other hand, NF-κB protects cells by inhibiting the accumulation of reactive oxygen species by activating transcription of antioxidant proteins (Sakon et al, 2003), and upregulates the transcription factor of an anti-inflammatory cytokine IL-10 by upregulating HO-1 expression (Lee and Chau, 2002; Saraiva and O’Garra, 2010; Tai et al, 2009). NF-κB has been found to be important for the induction of HO-1 expression in response to diverse stimuli, such as TGF-β-1, TNF-α, and LPS (Malaguarnera et al, 2005; Ning et al, 2002). The results of this study also show that NF-κB activation is essential for DHA-stimulated HO-1 expression, based on the fact that NF-κB inhibitors antagonized DHA-induced HO-1 expression. Furthermore, DHA induced increases in IKKα/β phosphorylation, IκBα phosphorylation, IκBα degradation, and p65 Ser536 phosphorylation. These findings suggest that NF-κB signaling serves a multifaceted role to control the maintenance of tissue inflammation homeostasis (Pasparakis, 2009).
DHA is a major structural component of phospholipids in neuronal cell membranes, whereas EPA is present in neuronal cell membranes in a very small amount (McNamara and Carlson, 2006). Therefore, it has been proposed that DHA is more important in brain functioning than EPA (Peet and Stokes, 2005). However, EPA, rather than DHA, seems to be the effective component when treating clinical depression in published studies (Freeman et al, 2006; Lin and Su, 2007). The contradiction between theoretical mechanisms and clinical studies, however, is not supported by our current findings from cellular models. Specifically, our study has shown that DHA and EPA had similar effects on the regulation of inflammation and HO-1 expression. As the designs of previous clinical trials have focused on the augmentation effects of n-3 PUFAs by enrolling only depressed patients receiving antidepressant medications simultaneously (Freeman et al, 2006; Lin and Su, 2007), the antidepressant effect of DHA might be hidden if the effects of DHA and antidepressant medications are overlapped. According to the results of lower DHA levels in depression in our previous meta-analytic review (Lin et al, 2010), it is too early to exclude the antidepressant effects of DHA. Nevertheless, our findings should still be interpreted with caution. As the model of IFN-induced cellular changes is based on the neural inflammation theory of depression, the antidepressant effects of n-3 PUFAs found from this particular cellular model might not be generalized to clinical heterogeneity of depression.
In conclusion, our study elucidates the antioxidative and anti-inflammatory effects of DHA and the manner in which the regulatory molecular mechanisms of DHA-induced HO-1 expression operate through the PI-3 kinase/AKT and ERK signaling pathways by increasing IKKα/β phosphorylation, p65 Ser536 phosphorylation, NF-κB activation, and HO-1 protein expression in microglia. By connecting with increasing evidence pointing to inflammation dysfunction in depression and clinical studies supporting the antidepressant effects of omega-3 PUFAs, our results provide a novel implication of antidepressant mechanisms of omega-3 fatty acids.
References
Abraham NG, Kappas A (2008). Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127.
Bal-Price A, Brown GC (2001). Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 21: 6480–6491.
Bazan NG (2006). Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 29: 263–271.
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990). Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27: 229–237.
Block ML, Zecca L, Hong JS (2007). Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8: 57–69.
Brand A, Bauer NG, Hallott A, Goldbaum O, Ghebremeskel K, Reifen R et al (2010). Membrane lipid modification by polyunsaturated fatty acids sensitizes oligodendroglial OLN-93 cells against oxidative stress and promotes upregulation of heme oxygenase-1 (HSP32). J Neurochem 113: 465–476.
Carlezon Jr WA, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF (2005). Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry 57: 343–350.
Charney DS, Manji HK (2004). Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE 2004: re5.
Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH et al (1999). The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem 72: 879–882.
Chen GG, Liu ZM, Vlantis AC, Tse GM, Leung BC, van Hasselt CA (2004). Heme oxygenase-1 protects against apoptosis induced by tumor necrosis factor-alpha and cycloheximide in papillary thyroid carcinoma cells. J Cell Biochem 92: 1246–1256.
Choi AM, Alam J (1996). Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 15: 9–19.
Choi BM, Kim BR (2008). Upregulation of heme oxygenase-1 by brazilin via the phosphatidylinositol 3-kinase/Akt and ERK pathways and its protective effect against oxidative injury. Eur J Pharmacol 580: 12–18.
Crawford Jr GD, Le WD, Smith RG, Xie WJ, Stefani E, Appel SH (1992). A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci 12: 3392–3398.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56.
De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S (2008). Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem 105: 296–307.
Duman RS, Malberg J, Nakagawa S, D’Sa C (2000). Neuronal plasticity and survival in mood disorders. Biol Psychiatry 48: 732–739.
Duman RS, Malberg J, Thome J (1999). Neural plasticity to stress and antidepressant treatment. Biol Psychiatry 46: 1181–1191.
Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA et al (2009). Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. J Neurochem 110: 1863–1875.
Farooqui AA, Ong WY, Horrocks LA (2006). Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev 58: 591–620.
Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M et al (2006). Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 67: 1954–1967.
Hanisch UK, Kettenmann H (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394.
Hibbeln JR (1998). Fish consumption and major depression. Lancet 351: 1213.
Hill-Kapturczak N, Thamilselvan V, Liu F, Nick HS, Agarwal A (2001). Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am J Physiol Renal Physiol 281: F851–F859.
Hirafuji M, Machida T, Tsunoda M, Miyamoto A, Minami M (2002). Docosahexaenoic acid potentiates interleukin-1beta induction of nitric oxide synthase through mechanism involving p44/42 MAPK activation in rat vascular smooth muscle cells. Br J Pharmacol 136: 613–619.
Horrobin DF, Bennett CN (1999). Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot Essent Fatty Acids 60: 217–234.
Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP (2008). Omega-3 fatty acids on the forced-swimming test. J Psychiatr Res 42: 58–63.
Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H (2008). Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 432: 232–236.
Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS (2005). Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol 69: 41–48.
Kawashima A, Harada T, Kami H, Yano T, Imada K, Mizuguchi K (2009). Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3-kinase signaling in rat hippocampus and differentiated PC12 cells. J Nutr Biochem 21: 268–277.
Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY et al (2008). Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264. 7 macrophages. Biomed Pharmacother 62: 630–636.
Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K (2003). Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med 34: 1006–1016.
Krishnan V, Nestler EJ (2008). The molecular neurobiology of depression. Nature 455: 894–902.
Lands WE (1992). Biochemistry and physiology of n-3 fatty acids. FASEB J 6: 2530–2536.
Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG (1994). Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA 91: 5987–5991.
Le WD, Xie WJ, Appel SH (1999). Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res 56: 652–658.
Lee TS, Chau LY (2002). Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8: 240–246.
Li B, Zhang S, Zhang H, Nu W, Cai L, Hertz L et al (2008a). Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology (Berl) 201: 443–458.
Li J, Ramenaden ER, Peng J, Koito H, Volpe JJ, Rosenberg PA (2008b). Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J Neurosci 28: 5321–5330.
Li W, Xia J, Sun GY (1999). Cytokine induction of iNOS and sPLA2 in immortalized astrocytes (DITNC): response to genistein and pyrrolidine dithiocarbamate. J Interferon Cytokine Res 19: 121–127.
Lin CC, Chiang LL, Lin CH, Shih CH, Liao YT, Hsu MJ et al (2007). Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur J Pharmacol 560: 101–109.
Lin PY, Huang SY, Su KP (2010). A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry (in press).
Lin PY, Su KP (2007). A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 68: 1056–1061.
Lu DY, Tang CH, Liou HC, Teng CM, Jeng KC, Kuo SC et al (2007). YC-1 attenuates LPS-induced proinflammatory responses and activation of nuclear factor-kappaB in microglia. Br J Pharmacol 151: 396–405.
Lu DY, Tang CH, Yeh WL, Wong KL, Lin CP, Chen YH et al (2009). SDF-1alpha up-regulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and NF-kappaB-dependent pathway in microglia. Eur J Pharmacol 613: 146–154.
Lu XH, Bradley RJ, Dwyer DS (2004). Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogen-activated protein kinase p38. Brain Res 1011: 58–68.
Lu XH, Dwyer DS (2005). Second-generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/AKT, ERK, and pertussis toxin-sensitive pathways. J Mol Neurosci 27: 43–64.
Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE et al (2007). Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging 28: 845–855.
Maes M (2008). The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett 29: 287–291.
Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H (1996). Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord 38: 35–46.
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G et al (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24: 27–53.
Malaguarnera L, Imbesi R, Di RM, Scuto A, Castrogiovanni P, Messina A et al (2005). Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages. Int Immunopharmacol 5: 1458–1469.
Manji HK, Drevets WC, Charney DS (2001). The cellular neurobiology of depression. Nat Med 7: 541–547.
Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ (2003). A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry 160: 996–998.
McNamara RK, Carlson SE (2006). Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 75: 329–349.
McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE et al (2007). Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry 62: 17–24.
Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramauge M, Courtin F et al (2004). MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci 24: 207–216.
Miller AH, Maletic V, Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741.
Moon Y, Pestka JJ (2003). Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J Nutr Biochem 14: 717–726.
Nakao A, Kaczorowski DJ, Zuckerbraun BS, Lei J, Faleo G, Deguchi K et al (2008). Galantamine and carbon monoxide protect brain microvascular endothelial cells by heme oxygenase-1 induction. Biochem Biophys Res Commun 367: 674–679.
Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH (2006). Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 163: 1098–1100.
Ning W, Song R, Li C, Park E, Mohsenin A, Choi AM et al (2002). TGF-beta1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 283: L1094–L1102.
O’Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ (2009). Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol 12: 687–699.
Pasparakis M (2009). Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol 9: 778–788.
Peet M, Horrobin DF (2002). A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 59: 913–919.
Peet M, Murphy B, Shay J, Horrobin D (1998). Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 43: 315–319.
Peet M, Stokes C (2005). Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 65: 1051–1059.
Raison CL, Capuron L, Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24–31.
Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y et al (2003). NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909.
Saraiva M, O’Garra A (2010). The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181.
Saw CL, Huang Y, Kong AN (2010). Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol 79: 421–430.
Seti H, Leikin-Frenkel A, Werner H (2009). Effects of omega-3 and omega-6 fatty acids on IGF-I receptor signalling in colorectal cancer cells. Arch Physiol Biochem 115: 127–136.
Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD et al (2009). Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol 13: 257–263.
Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA (2005). Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 72: 211–218.
Song C, Leonard BE, Horrobin DF (2004a). Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress 7: 43–54.
Song C, Li X, Kang Z, Kadotomi Y (2007). Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology 32: 736–744.
Song C, Manku MS, Horrobin DF (2008). Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J Nutr 138: 954–963.
Song C, Phillips AG, Leonard BE, Horrobin DF (2004b). Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol Psychiatry 9: 630–638.
Su KP (2008). Mind-body interface: the role of n-3 fatty acids in psychoneuroimmunology, somatic presentation, and medical illness comorbidity of depression. Asia Pac J Clin Nutr 17 (Suppl 1): 151–157.
Su KP (2009). Biological mechanism of antidepressant effect of omega-3 fatty acids: how does fish oil act as a ‘mind-body interface′? Neurosignals 17: 144–152.
Su KP, Huang SY, Chiu CC, Shen WW (2003). Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol 13: 267–271.
Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC et al (2008). Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 69: 644–651.
Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC et al (2010). Phospholipase A2 and Cyclooxygenase 2 Genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry 67: 550–557.
Su KP, Shen WW, Huang SY (2000). Effects of polyunsaturated fatty acids on psychiatric disorders. Am J Clin Nutr 72: 1241.
Tai YH, Tsai RY, Lin SL, Yeh CC, Wang JJ, Tao PL et al (2009). Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology 110: 1379–1389.
Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS (2006). Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain 124: 77–86.
Tsoyi K, Kim HJ, Shin JS, Kim DH, Cho HJ, Lee SS et al (2008). HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal 20: 1839–1847.
Vareille M, Rannou F, Thelier N, Glasser AL, de ST, Martin C et al (2008). Heme oxygenase-1 is a critical regulator of nitric oxide production in enterohemorrhagic Escherichia coli-infected human enterocytes. J Immunol 180: 5720–5726.
Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R (2009). PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 34: 199–211.
Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS (2006). Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci 78: 2889–2897.
Yavin E, Brand A, Green P (2002). Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci 5: 149–157.
Zhang M, Jin W, Zhou X, Yu J, Lee AJ, Sun SC (2009). Deregulation of Tpl2 and NF-kappaB signaling and induction of macrophage apoptosis by the anti-depressant drug lithium. Cell Signal 21: 559–566.
Acknowledgements
The work was supported by the following grants: NSC 98-2627-B-039-003, NSC 98-2627-B-039-005, and NSC 98-2628-B-039-020-MY3 from the National Science Council in Taiwan; CMU97-336, CMU97-340, and CMU97-341 from the China Medical University in Taiwan; and the NARSAD Young Investigator Award in the United States. We thank Dr WD Le at the Baylor College of Medicine, Texas, USA, for providing the MES 23.5 cell line and Mr MC Tseng for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors report no biomedical financial interests or potential conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Lu, DY., Tsao, YY., Leung, YM. et al. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for Omega-3 Fatty Acids. Neuropsychopharmacol 35, 2238–2248 (2010). https://doi.org/10.1038/npp.2010.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.98
Keywords
This article is cited by
-
Study protocol: fish oil supplement in prevention of oxaliplatin-induced peripheral neuropathy in adjuvant colorectal cancer patients – a randomized controlled trial. (OxaNeuro)
BMC Cancer (2024)
-
Glucose increases proliferation and chemoresistance in chronic myeloid leukemia via decreasing antioxidant Properties of ω-3 polyunsaturated fatty acids in the presence of Iron
Molecular Biology Reports (2023)
-
The impact of maternal obesity on childhood neurodevelopment
Journal of Perinatology (2021)
-
A Walnut Diet in Combination with Enriched Environment Improves Cognitive Function and Affects Lipid Metabolites in Brain and Liver of Aged NMRI Mice
NeuroMolecular Medicine (2021)
-
Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells
Journal of Neuroinflammation (2018)