Abstract
The amygdala is a key structure in the pathophysiology of anxiety disorders, and a putative target for anxiolytic treatments. Selective serotonin reuptake inhibitors (SSRIs) and placebo seem to induce anxiolytic effects by attenuating amygdala responsiveness. However, conflicting amygdala findings have also been reported. Moreover, the neural profile of responders and nonresponders is insufficiently characterized and it remains unknown whether SSRIs and placebo engage common or distinct amygdala subregions or different modulatory cortical areas. We examined similarities and differences in the neural response to SSRIs and placebo in patients with social anxiety disorder (SAD). Positron emission tomography (PET) with oxygen-15-labeled water was used to assess regional cerebral blood flow (rCBF) in 72 patients with SAD during an anxiogenic public speaking task, before and after 6–8 weeks of treatment under double-blind conditions. Response rate was determined by the Clinical Global Impression-Improvement scale. Conjunction analysis revealed a common rCBF-attenuation from pre- to post-treatment in responders to SSRIs and placebo in the left basomedial/basolateral and right ventrolateral amygdala. This rCBF pattern correlated with behavioral measures of reduced anxiety and differentiated responders from nonresponders. However, nonanxiolytic treatment effects were also observed in the amygdala. All subgroups, including nonresponders, showed deactivation of the left lateral part of the amygdala. No rCBF differences were found between SSRI responders and placebo responders. This study provides new insights into the brain dynamics underlying anxiety relief by demonstrating common amygdala targets for pharmacologically and psychologically induced anxiety reduction, and by showing that the amygdala is functionally heterogeneous in anxiolysis.
Similar content being viewed by others
INTRODUCTION
Anxiety disorders, the most common mental disorders (Kessler et al, 2005), are associated with intense personal suffering (Kessler, 2003) and considerable societal costs (François et al, 2010). To be able to prevent and treat anxiety efficaciously, it is important to understand its neurobiological underpinnings. Subcortical regions such as the amygdala, hippocampus, brainstem, and hypothalamus, as well as cortical areas such as the insular, orbitofrontal (OFC), ventromedial prefrontal (vmPFC), and anterior cingulate (ACC) cortices have been suggested to be involved in the neuropathology of anxiety disorders (Shin and Liberzon, 2010). The OFC, vmPFC, and ACC are thought to have a significant modulatory role in the treatment of anxiety (Mathew et al, 2008) because of their involvement in affective processing and emotional regulation (Ochsner and Gross, 2005; Rosen and Levenson, 2009). The amygdala, because of its key role in fear learning and processing (Phelps and LeDoux, 2005; Shin and Liberzon, 2010), is commonly viewed as the central target for anxiolytic treatments (Mathew et al, 2008).
Selective serotonin reuptake inhibitors (SSRIs) are generally accepted as the first-line pharmacological treatment for anxiety disorders (Davidson, 2006; Ravindran and Stein, 2010). Animal models provide evidence for a modulatory effect of serotonin on amygdala firing (Cheng et al, 1998; Stutzmann et al, 1998) and amygdala-related behavior (Inoue et al, 2004). Human imaging studies also suggest that SSRIs regulate amygdala hyper-responsivity to threat-relevant stimuli in healthy participants (Harmer et al, 2006; Murphy et al, 2009), and in patients with social anxiety disorder (SAD; Furmark et al, 2002, 2005). However, an inherent difficulty of pharmaco-imaging trials is to separate the biomarkers that are specifically associated with clinical response from the nonspecific pharmacodynamic effects that occur regardless of symptom improvement. Despite the extensive use of SSRIs, it is widely recognized that a considerable number of patients fails to achieve adequate therapeutic response with this medication (Fredman et al, 2000; Van Ameringen et al, 2000). Knowledge regarding brain perfusion in SSRI-nonresponders stems mainly from prediction studies (Evans et al, 2006) that do not explore the neural profile associated with insufficient response. As far as we know, there are no imaging activation study of anxiety patients that have compared SSRI responders and nonresponders.
In addition, the neural pathways that are specific for SSRI response relative to placebo response are not well characterized in anxiety treatment. Concerns regarding the clinical effectiveness of SSRIs over placebo have been raised by meta-analyses (Fournier et al, 2010; Khan et al, 2002; Kirsch et al, 2008), and it is well documented that the magnitude of the placebo response, ie, the therapeutic effect of inert substances, causes difficulties in establishing the efficacy of several pharmacological agents (Khan and Bhat, 2008; Uhlenhuth et al, 1997). Expectations of clinical improvement, elicited by placebo, are thought to modulate several clinical outcomes (Colloca and Miller, 2011; Fuente-Fernández et al, 2004; Lidstone et al, 2010; Scott et al, 2007) and neurofunctionally, this might correspond to a general top–down modulatory process (Benedetti et al, 2006; Krummenacher et al, 2010; Petrovic et al, 2005; Zubieta and Stohler, 2009). At a neurophysiological level placebo responses are disorder specific (Benedetti et al, 2005). Studies reporting reduced activity in single neurons in the subthalamic nucleus of placebo responsive Parkinsonian patients (Benedetti et al, 2004) and reduced activity in the dorsal horn of the spinal cord of participants undergoing placebo analgesia (Eippert et al, 2009) are intriguing examples of how expectancies of improvement can modulate specific processes within the central nervous system. Moreover, studies suggest that placebo-induced clinical benefits seem to target specific brain regions similar to the ones recruited by active drugs (Faria et al, 2008; Petrovic et al, 2002). However, with regard to SSRIs there is a lack of studies examining the neurofunctional communalities and differences between placebo and SSRI response. In depressed patients, Mayberg et al (2002) reported a common pattern of glucose metabolism in neocortical and limbic–paralimbic brain regions for both placebo and SSRI responders, whereas Leuchter et al (2002) findings on prefrontal cordance suggest a distinct pathway for SSRI and placebo responders. Thus, it remains generally unclear whether successful placebos and SSRIs engage common or distinct brain patterns.
In patients with SAD, we previously reported that placebo response was accompanied by reduced anxiety-related activity in the amygdala (Furmark et al, 2008), resembling the neural effect of citalopram (Furmark et al, 2002, 2005) and cognitive-behavior therapy (CBT; Furmark et al, 2002). Exaggerated amygdala responsivity in SAD and other anxiety disorders may thus be normalized with successful treatment. However, even though amygdala hyper-responsiveness has been a consistent finding in neuroimaging activation studies of anxiety disorders (Domschke and Dannlowski, 2009), some counterintuitive results have been reported, such as increased amygdala activity in anxiety patients following successful CBT (Maslowsky et al, 2010), higher amygdala responsivity to aversive stimuli in healthy participants than in anxiety patients (Britton et al, 2005; Phan et al, 2006; Straube et al, 2006), and higher amygdala responsivity to emotionally neutral than to anxiogenic tasks (Britton et al, 2005; Kilts et al, 2006). Mixed amygdala findings have also been reported after acute administration of citalopram in healthy participants (Bigos et al, 2008; Murphy et al, 2009). Genetic variation and heterogeneity of the investigated samples, imaging methods, and experimental tasks might underlie these inconsistencies (Furmark et al, 2009). Mixed findings may also be related to the fact that the amygdala, which is usually treated as a single structure in neuroimaging studies, is functionally and anatomically heterogeneous as extensively demonstrated in animal studies (LeDoux, 2007). Hence, it is unlikely that the amygdala is a functionally homogeneous region in human anxiety. It is possible that SSRIs and placebo engage different subregions of the amygdala and/or different modulatory cortical activity patterns.
Impelled by the limited knowledge regarding the neurofunctional mechanisms underlying effective and ineffective treatments of clinical anxiety, the present study reanalyzed previously published data on placebo anxiolysis (Furmark et al, 2008) together with newly merged unpublished data on SSRIs, to examine the neural correlates of pharmacologically and psychologically induced anxiety reduction in patients with SAD. Neuroimaging data were extracted from three randomized double-blind placebo-controlled trials. Regional cerebral blood flow (rCBF) was assessed with oxygen-15-labeled water positron emission tomography (PET) during an anxiogenic public speaking task, before and after 6–8 weeks of treatment with either SSRIs or placebo. We used conjunction analysis and interaction contrasts to examine overlapping and unique neural changes in responders and nonresponders of both treatments. The general aim was to identify neural alterations specifically underlying symptom improvement with SSRIs and placebo. In addition, we sought to describe nonspecific neural effects of intervention such as non-anxiolytic pharmacodynamic influences and effects of repeated testing.
MATERIALS AND METHODS
Participants
A total of 72 patients who fulfilled the DSM-IV (American Psychiatric Association, 1994) criteria for SAD were included (see Table 1 for demographics). Participants were recruited through newspaper advertising. All the participants gave written informed consent, and the study was approved by the Swedish Medical Products Agency as well as the local ethics and radiation safety committees. A DSM-IV (American Psychiatric Association, 1994) diagnosis of SAD was established through the Structured Clinical Interview for DSM Disorders (First et al, 1998). Additional disorders were screened for, by use of the structured diagnostic MINI-interview (Sheehan et al, 1998). All patients had marked public speaking anxiety and SAD as the primary diagnosis. Forty-nine patients (68%) were diagnosed with the generalized subtype and 14 (19%) qualified for a comorbid anxiety disorder: specific phobia (n=9), generalized anxiety disorder (GAD; n=1), posttraumatic stress disorder (n=1), panic disorder (n=1), and specific phobia together with GAD (n=2).
Participants were excluded if they reported: (1) treatment of social anxiety in the past 6 months; (2) current serious or dominant psychiatric disorder other than SAD (eg, psychosis, major depressive or bipolar disorder), (3) chronic use of prescribed medication, (4) abuse of alcohol/narcotics, (5) pregnancy, (6) menopause, (7) left handedness, (8) previous PET-examination, and (9) any somatic or neurologic disorder that could be expected to influence the outcome of the study. Study details were registered at www.clinicaltrials.gov (identifier NCT00343707).
Clinical Trials
Data were extracted from three randomized double-blind clinical PET trials evaluating changes in rCBF following treatment with SSRIs and placebo (N=144). All trials were performed at the PET center in Uppsala with the collaboration of Quintiles AB Uppsala (Sweden), and GlaxoSmithKline Verona (Italy), during 2002–2005. The current study presents data on the pooled SSRI and placebo groups (three SSRI and three placebo groups) with novel comparative analyses and conjunction analyses of responders and nonresponders. Parts of the data set were included in previous publications, the SSRI and placebo groups from the first trial were included in Furmark et al (2005), whereas the placebo groups from the second and the third trials were included in Furmark et al (2008). There were three treatment arms in the first two trials and six arms in the third (Figure 1). One subject allocated to SSRIs discontinued medication after the first week due to initial nausea. Thus, analyses were based on 35 patients randomized to SSRIs consisting of 42 days of citalopram 40 mg, or 56 days of paroxetine 20 mg or 7.5 mg. There was a matching placebo arm for each SSRI arm. In the present analyses, the placebo arms are grouped into one cohort with a total of 37 patients—see Figure 1.
Flow diagram of subject eligibility from screening to statistical analysis. SSRIs, selective serotonin reuptake inhibitors.
There were no significant differences across trial 1–3 with regard to demographic characteristics and clinical variables according to χ2 and t-test (all P's>10). Likewise, when statistically comparing SSRI and placebo arms, as well as responders vs nonresponders, no significant differences were noted on demographic variables, or pretreatment clinical variables described in Table 1, including distributions for subtypes and comorbid/noncomorbid cases (all P's >0.10). To check for pretreatment differences in rCBF, we extracted mean values from all a priori regions of interest (ROI) and found no significant main effects of treatment regimen (ie, SSRIs/placebo), response (ie, responders/nonresponders), or regimen × response interactions (F1,68=0.005–2.05; P=0.99–0.12).
GlaxoSmithKline Verona supplied daily doses of study drugs and matching placebo for the three consecutive PET trials with a fixed dosing schedule performed under double-blind conditions. The first dose was given on the same day as the first PET examination and the final dose was administered 2–4 h before the final PET assessment after 42 or 56 days (post-treatment). Subjects did not receive any other form of treatment during the study period. Patients visited the clinic biweekly for assessments of compliance, side effects, and to receive new supplies of medication. After study completion, patients were offered further psychiatric consultation and pharmacotherapy.
Clinical Outcome Measurements
Response rate was determined by the Clinical Global Impression improvement item (CGI-I; Zaider et al, 2003) administered by an experienced psychiatrist. Patients having a score of 1 or 2 (very much or much improved) on the CGI-I at posttest were classified as responders, whereas those having scores of ⩾3 were considered to be nonresponders. Additional changes in the social anxiety symptomatology were assessed by the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987). Symptom experience during public speaking was assessed using the Spielberger State Anxiety Inventory (STAI-S; Spielberger et al, 1970) and subjective fear ratings was evaluated by a 0–100 (min–max) visual analog scale administered after each public speaking challenge. Measures were collected by independent and blinded assessors.
PET Assessments
The procedure for rCBF assessments, using [H215O]-PET, has been described elsewhere (Furmark et al, 2008). Before and after treatment, all patients were scanned during an anxiogenic public speaking task, ie, patients gave a 2½-min speech about a travel experience, while lying in the camera, surrounded by a silently observing audience of 6–8 persons. We used a 32-ring ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, USA), which enables acquisition of 63 contiguous planes of data with a distance of 2.46 mm, resulting in a total axial field of view of 155 mm.
Patients fasted for 3 h, and refrained from tobacco, alcohol, and caffeine 12 h before PET-investigations. Patients were positioned in the scanner with the head gently fixated and a venous catheter for tracer injections was inserted. Patients were instructed to prepare a 2½-min speech about a vacation or travel experience about 20 min before the initial emission scan. A 10-min transmission scan was performed using three retractable 68Ge rotating line sources. Following intravenous administration of the 15O-water tracer, ∼10 MBq/kg body weight, the emission scan (three 30 s frames, 3-D mode) started automatically when the bolus reached the brain (50 000 counts/s).
Immediately after tracer injection, patients were asked to start their speech and continue until they received instructions to stop. The speech was performed in presence of a standing silently observing audience of 6–8 persons. Patients were instructed to observe the audience while giving the speech that was being videotaped, to increase observational anxiety. Directly after the speech, state anxiety and fear ratings were obtained, ie, subjects rated how they felt during scans. The PET procedure was the same at post-treatment, but the speech topic was different.
Emission scans were reconstructed with a filter back projection using an 8 mm Hanning filter, resulting in a spatial resolution of about 5 mm in the field of view. Data were corrected for photon attenuation, decay, scattered radiation, and random coincidences. After reconstruction, a summation image of the three frames was made in order to obtain a better statistical reference for realignment and subsequent analyses.
Statistical Analysis
Behavioral data
Demographic and clinical data were analyzed using IBM SPSS 19 (IBM, Somer, NY, USA) with the significance level set at P<.05. Group differences in clinical response were evaluated using χ2-test and analyses of covariance (ANCOVA) with the pretreatment value as covariate.
PET data
Imaging data were analyzed using Statistical Parametric Mapping Software (SPM2 - Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab 7.3.0. (MathWorks, Natick, MA). PET images were realigned to correct for different positions between scans (pre vs post-treatment) and normalized to the Montreal Neurological Institute's (MNI) stereotactic template. Images were then smoothed using an 8 mm Gaussian kernel and scaled to give all scans the same global signal.
Effects on rCBF were evaluated at the voxel level (1 voxel=2 × 2 × 2 mm) with results described as xyz coordinates in MNI space. ROI analyses were performed with the following a priori regions: amygdala, hippocampus, hypothalamus, brainstem, insula, ACC, OFC, and vmPFC, defined by the Wake Forest University School of Medicine PickAtlas (Maldjian et al, 2003). In addition, exploratory whole-brain analyses were conducted. Statistically significant changes were examined at P<.05 corrected family wise (FWE) for multiple comparisons. Additional uncorrected P-values are reported for the amygdala region (Puncorr<.05). Anatomical localization was guided by the Talairach atlas (Talairach and Tournoux, 1988), the Talairach Daemon (Lancaster et al, 2000), and the brain atlas of Mai (Mai et al, 2004).
SPM-analyses were performed in six steps:
-
1)
Clinical effects on brain activity were initially investigated by contrasting rCBF changes, measured during public speaking before and after treatment, in responders and nonresponders within each treatment regimen (SSRI and placebo).
-
2)
To further evaluate the specific treatment effects of SSRI and placebo on the brain activity, between-group comparisons were conducted in the form of subgroup × time interaction analyses, eg, (SSRI responderspre–SSRI responderspost)–(SSRI nonresponderspre–SSRI nonresponderspost).
-
3)
To identify common rCBF changes in SSRI and placebo responders, the conjunction from the within-group contrasts was conducted, ie, (SSRI responders pre–post) and (placebo responders pre–post). Conjunction analysis presumes a common effect in all contrasts tested. To exclude the impact of nonspecific treatment effects, (eg, repeated testing), we used the corresponding rCBF changes in SSRI and placebo nonresponders as an exclusion mask. Thus, we only considered the voxels that were common for treatment responders, regardless of modality, while excluding voxels that changed with repeated testing but were unrelated to clinical improvement.
-
4)
To identify unspecific SSRI-neurophysiological effects (unrelated to symptom improvement), we computed the conjunction of both SSRI within-group contrasts, ie, (SSRI responders pre–post) and (SSRI nonresponders pre–post). To exclude the potential impact of repeated testing and symptom improvement, we used rCBF changes from pre- to post-treatment occurring within placebo responders and nonresponders as an exclusion mask.
-
5)
To track nonspecific rCBF changes occurring with repeated testing over time (pre–post), we estimated the conjunction of all subgroups, ie, (SSRI responders pre–post), (placebo responders pre–post), (SSRI nonresponders pre–post), and (placebo nonresponders pre–post).
-
6)
In addition, we extracted the maximum correspondent voxel values resulting from the conjunction analyses and correlated the rCBF change with corresponding measures of state anxiety (STAI-S, fear) and clinical severity (LSAS).
RESULTS
Behavioral Measures
After unblinding, it was revealed that there were 20 SSRI responders (57%) and 11 placebo responders (30%), ie, a higher response rate in the merged SSRI group as compared with placebo (χ2(1)=5.51, P=0.02). On clinical outcome measures, ANCOVAs confirmed significantly greater improvement in SSRI responders in comparison with SSRI nonresponders (LSAS: F1,32=30.24, P<.0001; STAI-S: F1,32=6.44, P=0.016; fear: F1,32=17.08, P=0.0002), whereas responders in the SSRI and placebo arms did not differ significantly (F1,28=0.34–2.18, P=0.56–0.15). Placebo responders were more improved than placebo nonresponders on the LSAS (F1,34=34.71, P<.0001) and STAI-S (F1,34=5.48, P=0.025) but not on fear ratings (F1,34=2.15, P=0.15). Finally, the two nonresponder subgroups did not differ on LSAS or fear ratings (F1,38=0.23–0.66, P=0.63–0.42), but a difference (SSRI>placebo) was noted on the STAI-S (F1,38=4.59, P=0.039)—see Table 1.
Regional Cerebral Blood Flow
Within-group treatment effects
Results from within-group analyses of responders and nonresponders to SSRI or placebo treatments are displayed in Supplementary Table 1. Notably, there was evidence of reduced amygdala response (pre>post) in all responder/nonresponder subgroups, suggesting that lowered amygdala responsivity is not exclusively related to clinical improvement. However, only SSRI and placebo responders exhibited reduced neural activity in the left basomedial/basolateral (BM/BLA) and right ventrolateral (VLA) amygdala subregions, suggesting that these areas are related to anxiety relief.
Between-group treatment effects
In the between-group analysis (Table 2) there was evidence, mostly at the uncorrected p-level, that amygdala rCBF was more attenuated (pre>post) in responders relative to nonresponders both in SSRI- and placebo-treated subjects. These effects were noted in the left BM/BLA and right VLA. A cluster with its peak in the left lateral amygdala showed a tendency for greater deactivation (pre>post) in SSRI nonresponders than in SSRI responders, suggesting that lowered responsivity in this amygdala subregion is unrelated to clinical improvement. Differential rCBF changes between responders and nonresponders to SSRIs were additionally noted in frontal regions (ACC, OFC, and Insula), whereas brainstem differences were observed between the two placebo groups (Table 2). There were no rCBF differences between responders to SSRIs and placebo.
Anxiolytic effects common to SSRIs and placebo
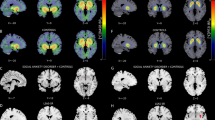
Conjunction analysis revealed a common reduction of amygdala activity in SSRI and placebo responders, with statistical peaks in the right VLA (x=28, y=−2, z=−26, Z=2.95, PFWE<.05) and left BM/BLA (x=−16, y=−6, z=−14, Z=2.49, Puncorr <.005; see Figures 2 and 3 and Supplementary Results). No overlapping rCBF changes were observed outside the amygdala region.
(a) Coronal images displaying decreased cerebral blood flow with treatment (pre–post) in three amygdala clusters, resultant from conjunction analyses. (1) The conjunction of selective serotonin reuptake inhibitor (SSRI) and placebo responders (with nonresponders to SSRIs and placebo as exclusive mask) showed overlapping amygdala deactivations with statistical peaks in the right ventrolateral and left basomedial/basolateral regions, indicating a common anxiolytic effect. (2) The conjunction of SSRI responders and SSRI nonresponders (with placebo responders and nonresponders as exclusive mask) revealed a common deactivation of the left lateral section of the amygdala, indicating a nonanxiolytic pharmacodynamic effect. (3) The conjunction of all groups, ie, SSRI and placebo responders/nonresponders, revealed a common deactivation of the left lateral amygdala, indicating an effect related to repeated testing over time. (b) Corresponding plots of percent change (±SE) in amygdala blood flow in responders and nonresponders in the three amygdala clusters related to (1) common anxiolytic effect of SSRIs and placebo; (2) non-anxiolytic pharmacodynamic effect of SSRIs, and (3) effect related to repeated testing.
The spatial extent of left (L) and right (R) amygdala clusters changing significantly with treatment. Red displays the common anxiolytic effect in selective serotonin reuptake inhibitor (SSRI) and placebo responders. Blue displays the nonanxiolytic pharmacodynamic effect of SSRIs noted in responders as well as nonresponders. Yellow displays the effect related to repeated testing noted in all four groups.
The rCBF change from pre- to post-treatment in the left BM/BLA and right VLA subregions of the amygdala (peak voxels) correlated with corresponding changes in LSAS (left: r=0.40, P<.001; right: r=0.34, P<.005), state anxiety (left: r=0.40, P<.001; right: r=0.22, P=0.062), and fear ratings (left: r=0.33 P<.005; right: r=0.21, P=0.065), suggesting that neural alterations in these regions underlie anxiety reduction regardless of treatment modality.
Non-anxiolytic effects
Pharmacodynamic effects of SSRIs:
The conjunction of SSRI responders and nonresponders revealed common rCBF decrement (pre>post) peaking in the left lateral amygdala (x=−26, y=0, z=−18; Z=3.38; PFWE<.005)—see Figures 2 and 3. The resultant amygdala peak value did not correlate significantly (P>0.10) with any behavioral anxiety measure, supportive of a symptom-unrelated pharmacodynamic effect.
Effects related to repeated testing:
The conjunction of all subgroups, including nonresponders, suggested a common deactivation (pre>post) with the statistical peak in the left lateral amygdala, (x=−28, y=0, z=−22; Z=2.14; Puncorr <.01)—see Figures 2 and 3. As expected, no significant correlations were observed between this amygdala region and behavioral anxiety measures (P>0.10).
DISCUSSION
The present study found evidence of common amygdala targets for effective SSRI and placebo treatments of SAD. Between-group and conjunction analyses showed that amygdala clusters with peak values in the left BM/BLA and right VLA sections were deactivated in SSRI responders and placebo responders alike, but not in nonresponders. Attenuated neural activity in these amygdala regions differentiated responders from nonresponders regardless of treatment modality, and correlated with behavioral measures of reduced anxiety.
Alleviation of social anxiety has been associated with lowered amygdala responsivity after successful CBT (Furmark et al, 2002), pharmacological (Furmark et al, 2002, 2005), and placebo treatments (Furmark et al, 2008). Interestingly, in the present study, we found no rCBF differences between responders to SSRI and placebo treatments. Together, these results are consistent with the notion that pharmacological and psychosocial treatments exert their beneficial effects, at least partly, by targeting the same brain regions (Faria et al, 2008). Cognitive factors such as expectations and beliefs are thought to have key roles in treatment outcomes (Colloca and Miller, 2011). Generally, pharmacological therapies and placebo share the cognitive factor associated with expectancies of symptom improvement. Hence, the common deactivation of the left BM/BLA and the right VLA sectors of the amygdala, observed only in responders, might result from patients’ belief in the effectiveness of the treatment, giving fuel to the speculation that SSRIs and placebo operate via similar belief-driven psychological mechanisms. Compelling evidence for expectancy-induced anxiety relief was found in anxious post-operative patients who after an open administration of diazepam reported significantly lower levels of anxiety in comparison to the ineffective hidden administration of benzodiazepine (Colloca et al, 2004). Concordantly, controversial meta-analytic studies in depression reported that placebos account for ∼75% of the improvement found in active pharmacological treatments (Kirsch and Sapirstein, 1998) and that the remaining portion could be explained by the use of inactive rather than active placebos (Kirsch et al, 2008; Moncrieff et al, 2004). However, our study cannot properly evaluate the impact of such psychological factors on neural systems because active placebos were not used and expectancies were not measured. Evidence from the animal literature, however, shows an inhibitory effect of SSRIs on amygdala responsivity and fear-related behaviors (Inoue et al, 2004; Izumi et al, 2006). Therefore, it is likely that the common response-related amygdala subregions constitute anxiolytic targets under the influence of both pharmacodynamic and psychological effects.
Even though certain parts of the amygdala were commonly deactivated in responders, and correlated with anxiety measures, the pattern within the broader amygdala region was complex. Decreased amygdala perfusion was noted in all subgroups including nonresponders. SSRI responders and SSRI nonresponders shared a common area of deactivation in the left lateral amygdala that was unrelated to anxiety relief, ie, a symptom-unrelated pharmacodynamic effect. Moreover, in the left, more ventral part of the lateral amygdala a common anxiety-unrelated deactivation was noted in all subgroups including nonresponders, reflecting repeated testing which is a confounding factor in clinical trials. Importantly, our results indicate that only certain parts of the amygdala mediate anxiety relief, whereas other amygdala deactivations reflect nonspecific effects of intervention.
Although usually treated as a single unit in human imaging studies, the amygdala is composed by multiple and functionally heterogeneous, but heavily interconnected, subnuclei (Aggleton, 1985; Pitkänen et al, 1997). Animal studies have reported differential distributions of the serotonin transporter protein (Rourke and Fudge, 2006) as well as serotonin–1A and other receptors (Pazos et al, 1987) across these subnuclei, which may underlie variation in treatment effects on amygdala firing. Damaging neurons of the central, basal, and lateral nuclei yields different mediation of fear-related behaviors in animals (Lázaro-Muñoz et al, 2010). Pharmacological studies have shown that the BLA and central amygdala, in particular, have distinct roles in the anxiolytic effect of benzodiazepines (Green and Vale, 1992). The highest concentration of benzodiazepine receptors appears to be in the BLA (Niehoff and Kuhar, 1983), which is involved in the modulation of inhibitory avoidance, characteristic for anxiety disorders (Bueno et al, 2005). Microinjection studies further support a central role of BLA in the anxiolytic effects of SSRIs (Inoue et al, 2004; Izumi et al, 2006). Moreover, stimulating BLA terminals in the central nucleus produced anxiolytic effects in a recent optogenetic study (Tye et al, 2011). Also in humans, BLA reactivity to unconscious stimuli has been shown to predict individual differences in trait anxiety (Etkin et al, 2009), making it a plausible target for anxiolytic treatments. Together these findings underscore the different roles of distinct amygdalar subnuclei in treatment response and the importance of BLA in anxiolysis.
The specific roles for amygdala subnuclei in conditioning (Morris et al, 2001) and social learning (Davis et al, 2010) have begun to be documented. For example, a recent fMRI study demonstrated that the right VLA was more reactive to negative faces, and was more resistant to habituation, in comparison with medial and dorsal amygdala loci (Davis et al, 2010). Broadly this is in line with our results, ie, the right VLA was tied to negative experience. Neuroimaging attempts to map interactions between distinct amygdala subregions and other brain regions also point to a functional complexity of the amygdala reflected by different connectivity patterns (Bach et al, 2011; Ressler, 2010; Roy et al, 2010). The question whether our observed anxiety-related amygdala subregions have similar or different connectivity networks in SSRI- and placebo-treated patients will be addressed in a separate report. Moreover, it is possible that the genetic control of neural activity differs across amygdala subregions, which in turn could be related to SSRI treatment outcome as previously suggested for placebo (Furmark et al, 2008). This will also be addressed in a separate report.
Although neural changes did not differ significantly between the responder subgroups, prefrontal differences were noted when comparing SSRI responders and nonresponders. Enhanced activity in OFC and ACC was observed in SSRI nonresponders, relative to responders, whereas the reverse pattern was noted in the insula. The meaning of these findings is unclear as the direction of the changes were generally contrary to what could be predicted from emotional regulation and processing theories (Goldin et al, 2008; Ochsner and Gross, 2005; Shin and Liberzon, 2010). However, other lines of research have shown that altered responsivity in these areas correlate with emotional experience or changes in ruminative and self-focused thoughts, ie, vulnerability factors in mood and anxiety disorders (Bar, 2009; Cooney et al, 2010). Placebo responders showed increased activity, relative to placebo nonresponders in the pons. This region has been reported as being involved in the opioid network affected by placebo analgesia (Petrovic et al, 2002).
The present study has some important limitations. First, to improve the generalizability of the SSRI results and to enable comparisons between responders and nonresponders with sufficient statistical power, data from patients treated with different SSRIs, different doses and different durations were pooled even though the clinical efficacy may vary (Telles-Correia et al, 2007). In particular, paroxetine 7.5 mg could be regarded as a subtherapeutic dose. Further analyses showed, however, that paroxetine 7.5 mg was neurophysiologically different from placebo but not deviant from paroxetine 20 mg in terms of reduced blood flow in the left lateral section of the amygdala that was demonstrated to be a general target for SSRIs (see Supplemental results). Statistically no behavioral or neural differences were found across the three SSRI arms, supporting that data could be merged. Second, because our paradigm did not include a neutral control task, we were not able to properly evaluate pretreatment amygdala reactivity and to exclude that attenuated amygdala activity after treatment reflects lowered trait-like activity. A previous study from our group suggested, however, that amygdala deactivation is related to state anxiety reduction (Furmark et al, 2005). Third, as we did not have a natural history or waiting list control group, it may be argued that our placebo results could be due to confounding variables such as spontaneous remission. However, this is unlikely as SAD appears to be a chronic condition that does not resolve without treatment (Yonkers et al, 2001). Concordantly, in a previous, similarly designed, PET study we did not observe clinical improvement or altered amygdala activity in waiting list controls (Furmark et al, 2002). Our randomized double-blind design protected against response biases and regression to the mean is also unlikely as there were no pretreatment differences between placebo responders and nonresponders on clinical variables or brain activity. Fourth, the PET technique has limited spatial resolution, which does not allow for a precise delineation of the amygdala subnuclei involved in anxiolytic and non-anxiolytic treatment responses. However, in spite of suboptimal anatomical precision and filtering (FWHM=8 mm), we were still able to separate functionally different clusters within the amygdaloid complex. Also, results were virtually the same when using a more narrow 6-mm filter. Importantly, the smallest interpeak distance between the reported anxiolytic and nonanxiolytic amygdala subregions was above 8 mm, indicating these clusters did not overlap. Moreover, PET has the advantage of being less sensitive to movement, which allows the implementation of an ecologically valid anxiogenic public speaking challenge that would be difficult to employ with more sensitive techniques such as fMRI. Nonetheless, high-resolution fMRI could be used with other anxiogenic paradigms to confirm and define, with better anatomical precision, the amygdala subnuclei that mediate reduced anxiety. The present findings need replication in other cohorts and further studies are also needed to clarify whether the neural effects are lateralized as our data suggest.
We conclude that only certain amygdala sections, the left BM/BLA and right VLA, were commonly deactivated in clinically improved patients treated with SSRIs or placebo, and may constitute brain targets that mediate successful anxiety reduction regardless of treatment modality. In contrast, deactivations of lateral amygdala sections, at least in the left hemisphere, appear to reflect nonspecific effects of intervention including nonanxiolytic pharmacodynamics and repeated testing. To our knowledge this is the first study showing that effective and ineffective treatments target different areas of the amygdala, shedding new lights on the neuromediators underlying anxiety relief. The amygdala is heterogeneous with respect to anxiolysis and should ideally be treated as such in human neuroimaging studies.
References
Aggleton JP (1985). A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res 57: 390–399.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM IV) 4th edn American Psychiatric Press: Washington, DC.
Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ (2011). Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. J Neurosci 31: 618–623.
Bar M (2009). A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci 13: 456–463.
Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I et al (2006). Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain 121: 133–144.
Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M et al (2004). Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci 7: 587–588.
Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (2005). Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–10402.
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008). Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33: 3221–3225.
Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I (2005). Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry 57: 832–840.
Bueno CH, Zangrossi H, Viana MB (2005). The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res 38: 1697–1701.
Cheng L-L, Wang S-J, Gean P-W (1998). Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 10: 2163–2172.
Colloca L, Lopiano L, Lanotte M, Benedetti F (2004). Overt vs covert treatment for pain, anxiety and Parkinson's disease. Lancet Neurol 3: 679–684.
Colloca L, Miller FG (2011). Role of expectations in health. Curr Opin Psychiatry 24: 149–155.
Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH (2010). Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10: 470–478.
Davidson JRT (2006). Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry 67: 20–26.
Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ (2010). Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex 20: 612–621.
Domschke K, Dannlowski U (2009). Imaging genetics of anxiety disorders. NeuroImage 15: 822–831.
Eippert F, Finsterbusch J, Bingel U, Büchel C (2009). Direct evidence for spinal cord involvement in placebo analgesia. Science 16: 404.
Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 6: 1361–1372.
Evans KC, Dougherty DD, Pollack MH, Rauch SL (2006). Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry 18: 33–42.
Faria V, Fredrikson M, Furmark T (2008). Imaging the placebo response: a neurofunctional review. Eur Neuropsychopharmacol 18: 473–485.
First M, Gibbon M, Spitzer R, Williams J (1998). SCID-I: Interview Protocol (in Swedish). Pilgrim Press: Stockholm.
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC et al (2010). Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303: 47–53.
François C, Despiégel N, Maman K, Saragoussi D, Auquier P (2010). Anxiety disorders, major depressive disorder and the dynamic relationship between these conditions: treatment patterns and cost analysis. J Med Econ 13: 99–109.
Fredman SJ, Fava M, Kienke AS, White CN, Nierenberg AA, Rosenbaum JF (2000). Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: A survey of current ‘next-step’ practices. J Clin Psychiatry 61: 403–408.
Fuente-Fernández R de la, Schulzer M, Stoessl AJ (2004). Placebo mechanisms and reward circuitry: clues from Parkinson's disease. Biol Psychiatry 56: 67–71.
Furmark T, Appel L, Henningsson S, Åhs F, Faria V, Linnman C et al (2008). A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J Neurosci 28: 13066–13074.
Furmark T, Appel L, Michelgård A, Wahlstedt K, Åhs F, Zancan S et al (2005). Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry 58: 132–142.
Furmark T, Henningsson S, Appel L, Åhs F, Linnman C, Pissiota A et al (2009). Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci 34: 30–40.
Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Långström B et al (2002). Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 59: 425–433.
Goldin PR, McRae K, Ramel W, Gross JJ (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63: 577–586.
Green S, Vale AL (1992). Role of amygdaloid nuclei in the anxiolytic effects of benzodiazepines in rats. Behav Pharmacol 3: 261–264.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820.
Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S et al (2004). Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol 497: 311–316.
Izumi T, Inoue T, Kitaichi Y, Nakagawa S, Koyama T (2006). Target brain sites of the anxiolytic effect of citalopram, a selective serotonin reuptake inhibitor. Eur J Psychopharmacol 534: 129–132.
Kessler RC (2003). The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr Scand 108: 19–27.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627.
Khan A, Bhat A (2008). Is the problem of a high placebo response unique to antidepressant trials? J Clin Psychiatry 69: 1979–1980.
Khan A, Leventhal RM, Khan SR, Brown WA (2002). Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 22: 40–45.
Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE et al (2006). The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology 31: 2243–2253.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (2008). Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5: e45.
Kirsch I, Sapirstein G (1998). Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prev Treat 6: 1–16.
Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G (2010). Prefrontal cortex modulates placebo analgesia. Pain 48: 368–374.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al (2000). Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131.
Lázaro-Muñoz G, LeDoux JE, Cain CK (2010). Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol Psychiatry 67: 1120–1127.
LeDoux J (2007). The Amygdala. Curr Biol 17: R868–R874.
Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M (2002). Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry 159: 122–129.
Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ et al (2010). Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry 67: 857–865.
Liebowitz MR (1987). Social phobia. Mod Probl Pharmacopsychiatry 22: 141–173.
Mai JK, Assheuer J, Paxinos G (2004). Atlas of the Human Brain. Elsevier Academic: San Diego.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239.
Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS et al (2010). A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adol Psychopharmacol 20: 105–111.
Mathew SJ, Price RB, Charney DS (2008). Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genetics 148: 89–98.
Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S et al (2002). The functional neuroanatomy of the placebo effect. Am J Psychiatry 159: 728–737.
Moncrieff J, Wessely S, Hardy R (2004). Active placebos vs antidepressants for depression. Cochrane Database Syst Rev 1: CD003012.
Morris JS, Buchel C, Dolan RJ (2001). Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. NeuroImage 13: 1044–1052.
Murphy SE, Norbury R, Sullivan UO, Cowen PJ, Harmer CJ (2009). Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194: 535–540.
Niehoff DL, Kuhar MJ (1983). Benzodiazepine receptors: localization in rat amygdala. J Neurosci 3: 2091–2097.
Ochsner KN, Gross JJ (2005). The cognitive control of emotion. Trends Cogn Sci 9: 242–249.
Pazos A, Probst A, Palacios JM (1987). Serotonin receptors in the human brain-III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21: 97–122.
Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M (2005). Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46: 957–969.
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002). Placebo and opioid analgesia. Imaging a shared neuronal network. Science 295: 1737–1740.
Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry 63: 184–192.
Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187.
Pitkänen A, Savander V, LeDoux JE (1997). Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523.
Ravindran LN, Stein MB (2010). The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry 71: 839–854.
Ressler KJ (2010). Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry 67: 1117–1119.
Rosen HJ, Levenson RW (2009). The emotional brain: combining insights from patients and basic science. Neurocase 15: 173–181.
Rourke HO, Fudge JL (2006). Distribution of serotonin transporter labeled fibers in distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry 60: 479–490.
Roy AK, Shehzad Z, Marqulies DS, Kelly AM, Uddin LQ, Gotimer K et al (2010). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage 45: 614–626.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK (2007). Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55: 325–336.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33.
Shin LM, Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 35: 169–191.
Spielberger CD, Gorsuch RL, Lushene RE (1970). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Straube T, Glauer M, Dilger S, Mentzel H-J, Miltner WHR (2006). Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage 29: 125–135.
Stutzmann GE, McEwen BS, LeDoux JE (1998). Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci 18: 9529–9538.
Talairach J, Tournoux P (1988). Co-Planar Stereotaxic Atlas of the Human Brain. Georg Thiene Verlag: Stuttgart, Germany.
Telles-Correia D, Guerreiro DF, Oliveira S, Figueira ML (2007). Differences between SSRI's pharmacokinetics and pharmacodynamics. Acta Med Port 20: 167–174.
Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H et al (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362.
Uhlenhuth EH, Matuzas W, Warner TD, Thompson PM (1997). Growing placebo response rate: the problem in recent therapeutic trials? Psychopharmacol Bull 33: 31–39.
Van Ameringen M, Mancini C, Farvolden P, Oakman J (2000). Drugs in development for social anxiety disorder: more to social anxiety than meets the SSRI. Expert Opin Investig Drugs 9: 2215–2231.
Yonkers KA, Dyck IR, Keller MB (2001). An eight-year longitudinal comparison of clinical course and characteristics of social phobia among men and women. Psychiatric Services 52: 637–643.
Zaider TI, Heimberg RG, Fresco DM, Scheiner FR, Liebowitz MR (2003). Evaluation of the clinical global impression scale among individuals with social anxiety disorder. Psychol Med 33: 611–622.
Zubieta JK, Stohler CS (2009). Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 1156: 198–210.
Acknowledgements
This work was supported by GlaxoSmithKline, the Swedish Research Council, the Swedish Council for Working Life and Social Research, and a grant from the Foundation of Science and Technology from the Portuguese Ministry of Science, Technology and Higher Education co-financed by the European Social funding. We thank all patients for their contribution to this study and the staff members of Uppsala PET centre and Quintiles for providing excellent research conditions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Massimo Bani, Paolo Bettica, and Emilio Merlo Pich were full-time employees at GlaxoSmithKline at the time of the design and conduct of this study. None of the other authors declare conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Faria, V., Appel, L., Åhs, F. et al. Amygdala Subregions Tied to SSRI and Placebo Response in Patients with Social Anxiety Disorder. Neuropsychopharmacol 37, 2222–2232 (2012). https://doi.org/10.1038/npp.2012.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.72
Keywords
This article is cited by
-
Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder
Translational Psychiatry (2022)
-
Nucleus accumbens volume as a predictor of anxiety symptom improvement following CBT and SSRI treatment in two independent samples
Neuropsychopharmacology (2020)
-
Developmental Pathways from Early Behavioral Inhibition to Later Anxiety: An Integrative Review of Developmental Psychopathology Research and Translational Implications
Adolescent Research Review (2019)
-
Neuroimaging Predictors and Mechanisms of Treatment Response in Social Anxiety Disorder: an Overview of the Amygdala
Current Psychiatry Reports (2018)
-
Mechanisms of the placebo effect in pain and psychiatric disorders
The Pharmacogenomics Journal (2016)