Key Points
-
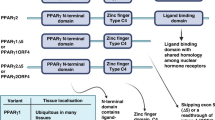
Peroxisome-proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear-hormone-receptor family. Three isotypes have been identified — PPARα, PPARβ/δ and PPARγ.
-
PPARs are activated by endogenous ligands — fatty acids and fatty-acid derivatives, for which they act as intracellular sensors — which leads to transcriptional regulation of pathways that are involved in lipid and glucose metabolism.
-
PPARα is the therapeutic target of the fibrates, which are widely used as hypolipidaemic compounds in the treatment of dyslipidaemia. PPARγ is a non-exclusive target of the thiazolidinediones, a new class of compounds with hypoglycaemic properties that are used to treat type II diabetes. Because of its involvement in lipid metabolism and skin homeostasis, it is likely that PPARβ/δ will become a therapeutic target in the near future.
-
Each PPAR isotype is associated with pathways that relate to carcinogenesis. Long-term activation of PPARα by peroxisome proliferators induces the development of hepatocarcinomas in rodent liver, but not in humans. PPARγ is thought to have overall anti-carcinogenic effects in many different cell types, due to its anti-proliferation, pro-differentiation and pro-apoptotic properties. PPARβ/δ is involved in the control of cell proliferation, cell differentiation and apoptosis, but its involvement in the development of tumours is unclear at present.
-
In the future, PPARγ, and perhaps PPARβ/δ, might become interesting therapeutic targets for the treatment of tumours. However, further investigation is needed at both the scientific and clinical levels.
Abstract
Peroxisome-proliferator-activated receptors (PPARs) are nuclear hormone receptors that mediate the effects of fatty acids and their derivatives at the transcriptional level. Through these pathways, PPARs can regulate cell proliferation, differentiation and survival, so controlling carcinogenesis in various tissues. But what are the links between each PPAR isotype and carcinogenesis and what is the relevance of these findings to human pathology and therapy?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
A unified nomenclature system for the nuclear receptor superfamily. Cell 97, 161–163 (1999).
Kersten, S. & Wahli, W. Peroxisome proliferator activated receptor agonists. EXS 89, 141–151 (2000).
Tan, N. S. et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 22, 5114–5127 (2002). This paper addresses the question of the role of fatty-acid-binding proteins in the cytoplasmic transport and nuclear delivery of PPAR ligands, which are hydrophobic molecules, to their cognate nuclear receptor.
Surapureddi, S. et al. Identification of a transcriptionally active peroxisome proliferator-activated receptor α-interacting cofactor complex in rat liver and characterization of PRIC285 as a coactivator. Proc. Natl Acad. Sci. USA 99, 11836–11841 (2002).
Mueller, E. et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor γ isoforms. J. Biol. Chem. 277, 41925–41930 (2002).
Krogsdam, A. M. et al. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor δ-mediated transactivation. Biochem. J. 363, 157–165 (2002).
Shi, Y., Hon, M. & Evans, R. M. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl Acad. Sci. USA 99, 2613–2618 (2002). This work indicates that PPARβ/δ interacts with co-repressors and competes with PPARα and PPARγ on PPAR response elements. PPARβ/δ could therefore act as a regulator of the expression of PPAR target genes, either through transcriptional activation or through competition with the two other PPAR isotypes and transcriptional repression.
Dowell, P. et al. Identification of nuclear receptor corepressor as a peroxisome proliferator-activated receptor α interacting protein. J. Biol. Chem. 274, 15901–15907 (1999).
Desvergne, B. & Wahli, W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20, 649–688 (1999).
Di-Poi, N., Tan, N. S., Michalik, L., Wahli, W. & Desvergne, B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 10, 721–733 (2002). First description of PPARβ/δ target genes, showing that PPARβ/δ controls the AKT1 survival pathway in keratinocytes. This paper presents a model for the anti-apoptotic role of PPARβ/δ.
Michalik, L. et al. PPAR expression and function during vertebrate development. Int. J. Dev. Biol. 46, 105–114 (2002).
Michalik, L., Desvergne, B. & Wahli W. Peroxisome proliferator-activated receptors β/δ: emerging roles for a previously neglected third family member. Curr. Opin. Lipidol. 14, 129–135 (2003).
Lock, E. A., Mitchell, A. M. & Elcombe, C. R. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annu. Rev. Pharmacol. Toxicol. 29, 145–163 (1989).
Vanden Heuvel, J. P. et al. Comprehensive analysis of gene expression in rat and human hepatoma cells exposed to the peroxisome proliferator WY14,643. Toxicol. Appl. Pharmacol. 188, 185–198 (2003).
Corton, J. C., Lapinskas, P. J. & Gonzalez, F. J. Central role of PPARα in the mechanism of action of hepatocarcinogenic peroxisome proliferators. Mutat. Res. 448, 139–151 (2000).
Boitier, E., Gautier, J. C. & Roberts, R. Advances in understanding the regulation of apoptosis and mitosis by peroxisome-proliferator activated receptors in pre-clinical models: relevance for human health and disease. Comp. Hepatol. 2, 3 (2003).
Issemann, I. & Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347, 645–650 (1990).
Lee, S. S. et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15, 3012–3022 (1995). This paper describes the phenotype of the Pparα -null mice, a clear demonstration that this receptor is the one mediating the effects of PP in rodent liver.
Peters, J. M., Cattley, R. C. & Gonzalez, F. J. Role of PPAR α in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis 18, 2029–2033 (1997).
Marsman, D. S., Cattley, R. C., Conway, J. G. & Popp, J. A. Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (Wy-14,643) in rats. Cancer Res. 48, 6739–6744 (1988).
Isenberg, J. S., Kolaja, K. L., Ayoubi, S. A., Watkins, J. B. 3rd & Klaunig, J. E. Inhibition of WY-14,643 induced hepatic lesion growth in mice by rotenone. Carcinogenesis 18, 1511–1519 (1997).
Peters, J. M. et al. Role of peroxisome proliferator-activated receptor α in altered cell cycle regulation in mouse liver. Carcinogenesis 19, 1989–1994 (1998).
Bayly, A. C., Roberts, R. A. & Dive, C. Suppression of liver cell apoptosis in vitro by the non-genotoxic hepatocarcinogen and peroxisome proliferator nafenopin. J. Cell Biol. 125, 197–203 (1994).
Oberhammer, F. A. & Qin, H. M. Effect of three tumour promoters on the stability of hepatocyte cultures and apoptosis after transforming growth factor-β 1. Carcinogenesis 16, 1363–1371 (1995).
Gill, J. H., James, N. H., Roberts, R. A. & Dive, C. The non-genotoxic hepatocarcinogen nafenopin suppresses rodent hepatocyte apoptosis induced by TGFβ1, DNA damage and Fas. Carcinogenesis 19, 299–304 (1998).
Gill, J. H., Brickell, P., Dive, C. & Roberts, R. A. The rodent non-genotoxic hepatocarcinogen nafenopin suppresses apoptosis preferentially in non-cycling hepatocytes but also elevates CDK4, a cell cycle progression factor. Carcinogenesis 19, 1743–1747 (1998).
Roberts, R. A. Non-genotoxic hepatocarcinogenesis: suppression of apoptosis by peroxisome proliferators. Ann. NY Acad. Sci. 804, 588–611 (1996).
Roberts, R. A., James, N. H., Woodyatt, N. J., Macdonald, N. & Tugwood, J. D. Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor α (PPAR α). Carcinogenesis 19, 43–48 (1998).
Reddy, J. K. & Rao, M. S. Oxidative DNA damage caused by persistent peroxisome proliferation: its role in hepatocarcinogenesis. Mutat. Res. 214, 63–68 (1989).
Yeldandi, A. V., Rao, M. S. & Reddy, J. K. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 448, 159–177 (2000).
Rusyn, I., Rose, M. L., Bojes, H. K. & Thurman, R. G. Novel role of oxidants in the molecular mechanism of action of peroxisome proliferators. Antioxid. Redox Signal. 2, 607–621 (2000).
Rusyn, I. et al. Phthalates rapidly increase production of reactive oxygen species in vivo: role of Kupffer cells. Mol. Pharmacol. 59, 744–750 (2001).
Ashby, J. et al. Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Hum. Exp. Toxicol. 13, S1–S117 (1994).
Cattley, R. C. et al. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul. Toxicol. Pharmacol. 27, 47–60 (1998).
Bentley, P. et al. Hepatic peroxisome proliferation in rodents and its significance for humans. Food Chem. Toxicol. 31, 857–907 (1993).
Scotto, C., Keller, J. M., Schohn, H. & Dauca, M. Comparative effects of clofibrate on peroxisomal enzymes of human (Hep EBNA2) and rat (FaO) hepatoma cell lines. Eur. J. Cell Biol. 66, 375–381 (1995).
Palmer, C. N., Hsu, M. H., Griffin, K. J., Raucy, J. L. & Johnson, E. F. Peroxisome proliferator activated receptor-α expression in human liver. Mol. Pharmacol. 53, 14–22 (1998).
Tugwood, J. D., Aldridge, T. C., Lambe, K. G., Macdonald, N. & Woodyatt, N. J. Peroxisome proliferator-activated receptors: structures and function. Ann. NY Acad. Sci. 804, 252–265 (1996).
Maloney, E. K. & Waxman, D. J. trans-Activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 161, 209–218 (1999).
Woodyatt, N. J., Lambe, K. G., Myers, K. A., Tugwood, J. D. & Roberts, R. A. The peroxisome proliferator (PP) response element upstream of the human acyl CoA oxidase gene is inactive among a sample human population: significance for species differences in response to PPs. Carcinogenesis 20, 369–372 (1999).
Lambe, K. G., Woodyatt, N. J., Macdonald, N., Chevalier, S. & Roberts, R. A. Species differences in sequence and activity of the peroxisome proliferator response element (PPRE) within the acyl CoA oxidase gene promoter. Toxicol. Lett. 110, 119–127 (1999).
Lake, B. G., Phillips, J. C., Linnell, J. C. & Gangolli, S. D. The in vitro hydrolysis of some phthalate diesters by hepatic and intestinal preparations from various species. Toxicol. Appl. Pharmacol. 39, 239–248 (1977).
Rhodes, C. et al. Comparative pharmacokinetics and subacute toxicity of di(2-ethylhexyl) phthalate (DEHP) in rats and marmosets: extrapolation of effects in rodents to man. Environ. Health Perspect. 65, 299–307 (1986).
Anderson, W. A., Castle, L., Scotter, M. J., Massey, R. C. & Springall, C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit. Contam. 18, 1068–1074 (2001).
Sarraf, P. et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nature Med. 4, 1046–1052 (1998).
Tanaka, T. et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 61, 2424–2428 (2001).
Osawa, E. et al. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 124, 361–367 (2003).
Saez, E. et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Med. 4, 1058–1061 (1998).
Lefebvre, A. M. et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Med. 4, 1053–1057 (1998).
Wasan, H. S., Novelli, M., Bee, J. & Bodmer, W. F. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc. Natl Acad. Sci. USA 94, 3308–3313 (1997).
Girnun, G. D. et al. APC-dependent suppression of colon carcinogenesis by PPARγ. Proc. Natl Acad. Sci. USA 99, 13771–13776 (2002). Paper that addresses the controversial role of Pparγ activation in colon carcinogenesis. The data presented indicate that the consequence of the activation of Pparγ on the development of colon tumours might depend on the existence of a previous mutation event in the Apc–β-catenin pathway.
Girnun, G. D. & Spiegelman, B. M. PPARγ ligands: taking Ppart in chemoprevention. Gastroenterology 124, 564–567 (2003).
Mueller, E. et al. Terminal differentiation of human breast cancer through PPARγ. Mol. Cell 1, 465–470 (1998).
Elstner, E. et al. Ligands for peroxisome proliferator-activated receptor γ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl Acad. Sci. USA 95, 8806–8811 (1998).
Mehta, R. G., Williamson, E., Patel, M. K. & Koeffler, H. P. A ligand of peroxisome proliferator-activated receptor γ, retinoids, and prevention of preneoplastic mammary lesions. J. Natl Cancer Inst. 92, 418–423 (2000).
Suh, N. et al. A new ligand for the peroxisome proliferator-activated receptor-γ (PPAR-γ), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 59, 5671–5673 (1999).
Clay, C. E., Monjazeb, A., Thorburn, J., Chilton, F. H. & High, K. P. 15-Deoxy-δ12,14-prostaglandin J2-induced apoptosis does not require PPARγ in breast cancer cells. J. Lipid Res. 43, 1818–1828 (2002).
Kubota, T. et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 58, 3344–3352 (1998).
Mueller, E. et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc. Natl Acad. Sci. USA 97, 10990–10995 (2000).
Hisatake, J. I. et al. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer Res. 60, 5494–5498 (2000).
Aldred, M. A. et al. Peroxisome proliferator-activated receptor γ is frequently downregulated in a diversity of sporadic nonmedullary thyroid carcinomas. Oncogene 22, 3412–3416 (2003).
Altiok, S., Xu, M. & Spiegelman, B. M. PPARγ induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 11, 1987–1998 (1997).
Shao, D. & Lazar, M. A. Peroxisome proliferator activated receptor γ, CCAAT/enhancer-binding protein α, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 272, 21473–21478 (1997).
Heaney, A. P., Fernando, M. & Melmed, S. PPAR-γ receptor ligands: novel therapy for pituitary adenomas. J. Clin. Invest. 111, 1381–1388 (2003).
Morrison, R. F. & Farmer, S. R. Role of PPARγ in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J. Biol. Chem. 274, 17088–17097 (1999).
Clay, C. E., Atsumi, G. I., High, K. P. & Chilton, F. H. Early de novo gene expression is required for 15-deoxy-Δ 12,14-prostaglandin J2-induced apoptosis in breast cancer cells. J. Biol. Chem. 276, 47131–47135 (2001).
Kitamura, S. et al. PPARγ agonists inhibit cell growth and suppress the expression of cyclin D1 and EGF-like growth factors in ras-transformed rat intestinal epithelial cells. Int. J. Cancer 94, 335–342 (2001).
Toyota, M. et al. Peroxisome proliferator-activated receptor γ reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1. Life Sci. 70, 1565–1575 (2002).
Qin, C. et al. Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Res. 63, 958–964 (2003).
Wang, C. et al. Inhibition of cellular proliferation through IκB kinase-independent and peroxisome proliferator-activated receptor γ-dependent repression of cyclin D1. Mol. Cell. Biol. 21, 3057–3070 (2001).
Gupta, R. A., Brockman, J. A., Sarraf, P., Willson, T. M. & DuBois, R. N. Target genes of peroxisome proliferator-activated receptor γ in colorectal cancer cells. J. Biol. Chem. 276, 29681–29687 (2001).
Gupta, R. A. et al. Peroxisome proliferator-activated receptor γ and transforming growth factor-β pathways inhibit intestinal epithelial cell growth by regulating levels of TSC-22. J. Biol. Chem. 278, 7431–7438 (2003).
Chen, G. G. et al. Apoptosis induced by activation of peroxisome-proliferator activated receptor-γ is associated with Bcl-2 and NF-κB in human colon cancer. Life Sci. 70, 2631–2646 (2002).
Satoh, T. et al. Activation of peroxisome proliferator-activated receptor-γ stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene 21, 2171–2180 (2002).
Patel, L. et al. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr. Biol. 11, 764–768 (2001).
Farrow, B. & Evers, B. M. Activation of PPARγ increases PTEN expression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 301, 50–53 (2003).
Xin, X., Yang, S., Kowalski, J. & Gerritsen, M. E. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 274, 9116–9121 (1999).
Tontonoz, P. et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc. Natl Acad. Sci. USA 94, 237–241 (1997).
Demetri, G. D. et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc. Natl Acad. Sci. USA 96, 3951–3956 (1999). Paper that describes the striking beneficial effects that are observed after PPARγ agonists are given to treat patients suffering from liposarcoma. These data indicate that troglitazone is a promising drug in the treatment of certain tumours.
Theocharis, S. et al. Expression of peroxisome proliferator activated receptor-γ in non-small cell lung carcinoma: correlation with histological type and grade. Lung Cancer 36, 249–255 (2002).
Gupta, R. A. & Dubois, R. N. Controversy: PPARγ as a target for treatment of colorectal cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G266–G269 (2002).
Dreyer, C. et al. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68, 879–887 (1992).
Kliewer, S. A. et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl Acad. Sci. USA 91, 7355–7359 (1994).
Amri, E. Z., Bonino, F., Ailhaud, G., Abumrad, N. A. & Grimaldi, P. A. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J. Biol. Chem. 270, 2367–2371 (1995).
Matsuo, H. & Strauss, J. F. 3rd. Peroxisome proliferators and retinoids affect JEG-3 choriocarcinoma cell function. Endocrinology 135, 1135–1145 (1994).
Jow, L. & Mukherjee, R. The human peroxisome proliferator-activated receptor (PPAR) subtype NUC1 represses the activation of hPPAR α and thyroid hormone receptors. J. Biol. Chem. 270, 3836–3840 (1995).
He, T. C., Chan, T. A., Vogelstein, B. & Kinzler, K. W. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99, 335–345 (1999). Important report on the identification of PPARβ/δ as a potential target of the APC–β-catenin tumour-suppressor pathway. This paper also indicates that the activity COX2 in colon tumours might provide PPAR ligands.
Shao, J., Sheng, H. & DuBois, R. N. Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 62, 3282–3288 (2002).
Gupta, R. A. et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proc. Natl Acad. Sci. USA 97, 13275–13280 (2000).
Park, B. H., Vogelstein, B. & Kinzler, K. W. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proc. Natl Acad. Sci. USA 98, 2598–2603 (2001). Based on a model of grafts using PPARbβ/δ wild-type or null cells, this paper shows that PPARβ/δ activity can affect tumorigenesis and indicates that PPARβ/δ inhibitors could inhibit tumour growth.
Barak, Y. et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc. Natl Acad. Sci. USA 99, 303–308 (2002).
Jaeckel, E. C. et al. Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor δ with head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 127, 1253–1259 (2001).
Tong, B. J. et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-δ in human endometrial adenocarcinoma. Neoplasia 2, 483–490 (2000).
Suchanek, K. M., May, F. J., Lee, W. J., Holman, N. A. & Roberts-Thomson, S. J. Peroxisome proliferator-activated receptor β expression in human breast epithelial cell lines of tumorigenic and non-tumorigenic origin. Int. J. Biochem. Cell Biol. 34, 1051–1058 (2002).
Michalik, L. et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol. 154, 799–814 (2001).
Tan, N. S. et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes Dev. 15, 3263–3277 (2001).
Peters, J. M. et al. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ). Mol. Cell. Biol. 20, 5119–5128 (2000).
Hao, C. M., Redha, R., Morrow, J. & Breyer, M. D. Peroxisome proliferator-activated receptor δ activation promotes cell survival following hypertonic stress. J. Biol. Chem. 277, 21341–21345 (2002).
Hatae, T., Wada, M., Yokoyama, C., Shimonishi, M. & Tanabe, T. Prostacyclin-dependent apoptosis mediated by PPAR δ. J. Biol. Chem. 276, 46260–46267 (2001).
Hellemans, K. et al. Peroxisome proliferator-activated receptor-β signaling contributes to enhanced proliferation of hepatic stellate cells. Gastroenterology 124, 184–201 (2003).
Zhang, J. et al. Peroxisome proliferator-activated receptor δ is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J. Biol. Chem. 277, 11505–11512 (2002).
Vosper, H. et al. The peroxisome proliferator-activated receptor δ promotes lipid accumulation in human macrophages. J. Biol. Chem. 276, 44258–44265 (2001).
Saluja, I., Granneman, J. G. & Skoff, R. P. PPAR δ agonists stimulate oligodendrocyte differentiation in tissue culture. Glia 33, 191–204 (2001).
Dannenberg, A. J. & DuBois, R. N. COX–2. A new target for cancer prevention and treatment (Karger, New Brunswick, 2003).
Purdue, P. E. & Lazarow, P. B. Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 17, 701–752 (2001).
Kersten, S., Desvergne, B. & Wahli, W. Roles of PPARs in health and disease. Nature 405, 421–424 (2000).
Deeb, S. S. et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genet. 20, 284–287 (1998).
Ristow, M., Muller-Wieland, D., Pfeiffer, A., Krone, W. & Kahn, C. R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N. Engl. J. Med. 339, 953–959 (1998).
Barroso, I. et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880–883 (1999).
Palakurthi, S. S., Aktas, H., Grubissich, L. M., Mortensen, R. M. & Halperin, J. A. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Res. 61, 6213–6218 (2001).
Baek, S. J., Wilson, L. C., Hsi, L. C. & Eling, T. E. Troglitazone, a peroxisome proliferator-activated receptor γ (PPAR γ) ligand, selectively induces the early growth response-1 gene independently of PPAR γ. A novel mechanism for its anti-tumorigenic activity. J. Biol. Chem. 278, 5845–5853 (2003).
Sarraf, P. et al. Loss-of-function mutations in PPAR γ associated with human colon cancer. Mol. Cell 3, 799–804 (1999).
Kroll, T. G. et al. PAX8–PPARγ1 fusion oncogene in human thyroid carcinoma. Science 289, 1357–1360 (2000).
Marques, A. R. et al. Expression of PAX8–PPAR γ1 rearrangements in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 87, 3947–3952 (2002).
Nikiforova, M. N. et al. RAS point mutations and PAX8–PPAR γ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 88, 2318–2326 (2003).
Cheung, L. et al. Detection of the PAX8–PPAR γ fusion oncogene in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 88, 354–357 (2003).
Ikezoe, T. et al. Mutational analysis of the peroxisome proliferator-activated receptor γ gene in human malignancies. Cancer Res. 61, 5307–5310 (2001).
Brockman, J. A., Gupta, R. A. & Dubois, R. N. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology 115, 1049–1055 (1998).
Asou, H. et al. Growth inhibition of myeloid leukemia cells by troglitazone, a ligand for peroxisome proliferator activated receptor γ, and retinoids. Int. J. Oncol. 15, 1027–1031 (1999).
Han, S. W., Greene, M. E., Pitts, J., Wada, R. K. & Sidell, N. Novel expression and function of peroxisome proliferator-activated receptor γ (PPARγ) in human neuroblastoma cells. Clin. Cancer Res. 7, 98–104 (2001).
Koga, H. et al. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology 33, 1087–1097 (2001).
Wakino, S. et al. Peroxisome proliferator-activated receptor γ ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. J. Biol. Chem. 275, 22435–22441 (2000).
Takahashi, N. et al. Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Lett. 455, 135–139 (1999).
Ohta, K., Endo, T., Haraguchi, K., Hershman, J. M. & Onaya, T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 86, 2170–2177 (2001).
Houston, K. D. et al. Inhibition of proliferation and estrogen receptor signaling by peroxisome proliferator-activated receptor γ ligands in uterine leiomyoma. Cancer Res. 63, 1221–1227 (2003).
Roth, A. D. et al. PPAR γ activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J. Neurosci. Res. 72, 425–435 (2003).
Tontonoz, P., Nagy, L., Alvarez, J. G., Thomazy, V. A. & Evans, R. M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998).
Chang, T. H. & Szabo, E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Res. 60, 1129–1138 (2000).
Yang, W. L. & Frucht, H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis 22, 1379–1383 (2001).
Chinetti, G. et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 273, 25573–25580 (1998).
Padilla, J., Kaur, K., Cao, H. J., Smith, T. J. & Phipps, R. P. Peroxisome proliferator activator receptor-γ agonists and 15-deoxy-Δ(12,14)(12,14)-PGJ(2) induce apoptosis in normal and malignant B-lineage cells. J. Immunol. 165, 6941–6948 (2000).
Toyoda, M. et al. A ligand for peroxisome proliferator activated receptor γ inhibits cell growth and induces apoptosis in human liver cancer cells. Gut 50, 563–567 (2002).
Keelan, J. A. et al. 15-Deoxy-Δ(12,14)-prostaglandin J(2), a ligand for peroxisome proliferator-activated receptor-γ, induces apoptosis in JEG3 choriocarcinoma cells. Biochem. Biophys. Res. Commun. 262, 579–585 (1999).
Okuno, A. et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 101, 1354–1361 (1998).
Herbert, B. S. et al. A peroxisome proliferator-activated receptor-γ agonist and the p53 rescue drug CP-31398 inhibit the spontaneous immortalization of breast epithelial cells. Cancer Res. 63, 1914–1919 (2003).
Acknowledgements
The authors wish to thank L. Gelman for critical reading of this manuscript. The work done in the authors' laboratory was supported by the Swiss National Science Foundation (grants to W.W. and to B.D.), the Etat de Vaud and the Human Frontier Science Program Organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
LocusLink
OMIM
Glossary
- PEROXISOMES
-
Organelles that are found in all organisms and in various cell types. They are involved in many different functions, among which β-oxidation of long-chain fatty acids and H2O2-based respiration are the most prominent.
- LIPOXYGENASES
-
5-, 12-, and 15-lipoxygenases are enzymes that catalyse a key step in the production of leukotrienes in platelets, macrophages, mastocytoma cells and leukocytes.
- CYCLOOXYGENASES
-
(COXs). These catalyse a key step in the conversion of 20-carbon polyunsaturated fatty acids — such as arachidonic acid — to prostaglandins. COX1 is constitutively expressed in most tissues, but COX2 is induced by pathophysiological conditions such as tumorigenesis or inflammatory situations. COX2 is now recognized as an interesting target for the treatment of cancers.
- LIPOPROTEIN
-
Association of proteins with triglycerides, phospholipids and cholesterol. Lipoproteins are produced in the liver and serve as lipid carriers in the blood.
- KERATINOCYTE
-
The main cell type of the epidermis, the uppermost layer of the skin. Basal keratinocytes are responsible for the renewal of the epidermis. The daughter keratinocytes then undergo a vectorial specific differentiation programme, while migrating to the top of the epidermis, where they finally die and desquamate.
- DYSLIPIDAEMIAS
-
The term lipidaemia refers to the circulating level of total lipids, including free fatty acids and lipoproteins. Dyslipidaemias are pathological disorders that affect normal lipidaemia, usually corresponding to high blood lipid levels that are a high risk factor for cardiovascular diseases.
- COLITIS
-
Inflammatory state of the colon, possibly due to a single cause such as bacterial infection.
Rights and permissions
About this article
Cite this article
Michalik, L., Desvergne, B. & Wahli, W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4, 61–70 (2004). https://doi.org/10.1038/nrc1254
Issue Date:
DOI: https://doi.org/10.1038/nrc1254
This article is cited by
-
PPARG activation promotes the proliferation of colorectal cancer cell lines and enhances the antiproliferative effect of 5-fluorouracil
BMC Cancer (2024)
-
Predicting the molecular mechanism-driven progression of breast cancer through comprehensive network pharmacology and molecular docking approach
Scientific Reports (2023)
-
Phenotypic plasticity and genetic control in colorectal cancer evolution
Nature (2022)
-
PPARɣ drives IL-33-dependent ILC2 pro-tumoral functions
Nature Communications (2021)
-
Fibroblast growth factor receptor 3 alterations and response to immune checkpoint inhibition in metastatic urothelial cancer: a real world experience
British Journal of Cancer (2021)