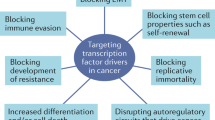
Key Points
Summary
-
Signalling proteins, which are often mutated in cancer, change transcription patterns.
-
Many more signalling proteins are affected in cancer than transcription factors, electing transcription factors as cogent targets.
-
One or more latent cytoplasmic transcription factors (such as STATs, NF-κB, β-catenin and Notch intracellular domain (NICD)) have increased activity in most human cancers, and in many cases prevent apoptosis of cancer cells.
-
Necessary physical interaction among transcription factors and cofactors in the nucleus affords selective sites of potential drug action.
-
Should pharmacology of transcription-factor inhibition be the wave of the future? It might be difficult, but it should not be impossible.
Abstract
A limited list of transcription factors are overactive in most human cancer cells, which makes them targets for the development of anticancer drugs. That they are the most direct and hopeful targets for treating cancer is proposed, and this is supported by the fact that there are many more human oncogenes in signalling pathways than there are oncogenic transcription factors. But how could specific transcription-factor activity be inhibited?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Varmus, H. E. Oncogenes and transcriptional control. Science 238, 1337–1339 (1987).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Vogt, P. K. Jun, the oncoprotein. Oncogene 20, 2365–2377 (2001).The discoverer of JUN presents an excellent summary of the first nuclear oncogene.
Futreal, P. A. et al. Cancer and genomics. Nature 409, 850–852 (2001).
Green, D. R. & Evan, G. I. A matter of life and death. Cancer Cell 1, 19–30 (2002).Authoritative account of the importance of apoptosis in cancer.
Gibbs, J. B. Mechanism-based target identification and drug discovery in cancer. Science 287, 1969–1973 (2000).
Biederer, C., Ries, S., Bandts, C. H. & McCormick, F. Replication-selective viruses for cancer therapy. J. Mol. Med. 80, 163–175 (2002).
McCormick, F. Cancer gene therapy: fringe or cutting edge? Nature Rev. Cancer 1, 130–141 (2001).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).Widely quoted summary of the requirements to make a human cancer cell.
Bachur, N. R. in Encyclopedia of Cancer 2nd edn Vol. 1 (ed. Bertino, J. R.) 57–61 (Academic Press, 2002).
Tilley, W. D., Clarke, C. L., Birrell, S. N. & Bruchovsky, N. Hormones and cancer: new insights, new challenges. Trends Endocrinol. Metab. 12, 186–188 (2001).
Barnes, P. J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. 94, 557–572 (1998).
Brivanlou, A. H. & Darnell, J. E. Jr., Signal transduction and the control of gene expression. Science 295, 813–818 (2002).
Vogt, P. K., Bos, T. J. & Doolittle, R. F. Homology between the DNA-binding domain of the GCN4 regulatory protein of yeast and the carboxyl-terminal region of a protein coded for by the oncogene jun. Proc. Natl Acad. Sci. USA 84, 3316–3319 (1987).
Derijard, B. et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76, 1025–1037 (1994).
Denhardt, D. T. Oncogene-initiated aberrant signaling engenders the metastatic phenotype: synergistic transcription factor interactions are targets for cancer therapy. Crit. Rev. Oncog. 7, 261–291 (1996).
Stark, G. R., Kerrr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Levy, D. & Darnell, J. E. Jr., STATs: transcriptional control and biologic impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002).Most up-to-date review of the STAT protein family, which is important in cancer.
Darnell, J. E. Jr., STATs and gene regulation. Science 277, 1630–1635 (1997).
Groner, B. et al. Regulation of the trans-activation potential of STAT5 through its DNA-binding activity and interactions with heterologous transcription factors. Growth Horm. IGF Res. 10 (Suppl. B), S15–S20 (2000).
Aittomaki, S. et al. Cooperation among Stat1, glucocorticoid receptor, and PU.1 in transcriptional activation of the high-affinity Fc gamma receptor I in monocytes. J. Immunol. 164, 5689–5697 (2000).
Look, D. C., Pelletier, M. R., Tidwell, R. M., Roswit, W. T. & Holtzman, M. J. Stat1 depends on transcriptional synergy with Sp1. J. Biol. Chem. 270, 30264–30267 (1995).
Zhang, X., Wrzeszczynaska, M. H., Horvath, C. M. & Darnell, J. E. Jr., Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol. Cell. Biol. 19, 7138–7146 (1999).
Starr, R. & Hilton, D. J. Negative regulation of the JAK/STAT pathway. Bioessays 21, 47–52 (1999).Authoritative summary of how cells switch off this important signalling pathway.
Aoki, N. & Matsuda, T. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 16, 58–69 (2002).
Shuai, K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene 19, 2638–2644 (2000).
Yu, C. L. et al. Enhanced DNA-binding of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269, 81–83 (1995).
Bowman, T., Garcia, R., Turkson, J. & Jove, R. STATs in oncogenesis. Oncogene 19, 2474–2488 (2000).
Bromberg, J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Takeda, K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell- specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 (1998).
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Shen, Y., Devgan, G., Darnell, J. E. Jr, & Bromberg, J. F. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc. Natl Acad. Sci. USA 98, 1543–1548 (2001).
Lacaronique, V. et al. A TEL–JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278, 1309–1312 (1997).
Lacronique, V. et al. Transforming properties of chimeric TEL–JAK proteins in Ba/F3 cells. Blood 95, 2076–2083 (200).
Song, J. I. & Grandis, J. R. STAT signaling in head and neck cancer. Oncogene 19, 2489–2495 (2000).Importance of persistently active STAT3 in human cancer.
Catlett-Falcone, R. et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999).
Yoshikawa, H. et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nature Genet. 28, 29–35 (2001).
Zhang, Q. et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immun. 168, 466–474 (2002).References 36–38 provide evidence of the importance of STAT3 in clinical cancer.
Perkins, N. D. The Rel/NF-κB family: friend and foe. Trends Biochem. Sci. 25, 434–440 (2000).
Israel, A. IκB kinase all zipped up. Nature 388, 519–521 (1997).
Sen, R. & Baltimore, D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 47, 921–928 (1986).
Tam, W. F., Wang, W. & Sen, R. Cell-specific association and shuttling of IκBα provides a mechanism for nuclear NF-κB in B lymphocytes. Mol. Cell. Biol. 21, 4837–4846 (2001).
Song, H. Y., Rothe, M. & Goeddel, D. V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl Acad. Sci. USA 93, 6721–6725 (1996).
Thanos, D. & Maniatis, T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83, 1091–1101 (1995).Early description of the protein complex that functions as the enhanceosome.
Rayet, B. & Gelinas, C. Aberrant REL/NF-κB genes and activity in human cancer. Oncogene 18, 6938–6947 (1999).
Ni, H. et al. Analysis of expression of nuclear factor B (NF-κB) in multiple myeloma: downregulation of NF-κB induces apoptosis. Br. J. Haematol. 115, 279–286 (2001).
Izban, K. F. et al. Characterization of NF-κB expression in Hodgkin's disease: inhibition of constitutively expressed NF-κB results in spontaneous caspase-independent apoptosis in Hodgkin and Reed–Sternberg cells. Mod. Pathol. 14, 297–310 (2001).
Kim, D. W. et al. Activation of NF-κB/REL occurs early during neoplastic transformation of mammary cells. Carcinogenesis 21, 871–879 (2000).
Tai, D. I. et al. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer 89, 2274–2281 (2000).
Taipale, J. & Beachy, P. A. The Hedgehog and Wnt signalling pathways in cancer. Nature 411, 349–354 (2001).Excellent review of two signalling pathways and how they might connect to human cancer.
Barish, G. D. & Williams, B. O. in Signaling Networks and Cell Cycle Control (ed. Gutkind, J. S) 53–82 (2000).
Kolligs, F. T. et al. Gamma-catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of beta-catenin. Genes Dev. 14, 1319–1331 (2000).
Wong, C. M., Fan, S. T. & Ng, I. O. L. Beta-catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer 92, 136–145 (2001).
van de Wetering, M. et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997).
Kramps, T. et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109, 47–60 (2002).
Parker, D. S., Jemison, J. & Cadigan, K. M. Nucleotide Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565–2576 (2002).
Thompson, B., Townsley, F., Rosin-Arbesfeld, R., Musisi, H. & Bienz, M. A new nuclear component of the Wnt signalling pathway. Nature Cell Biol. 4, 367–373 (2002).
Barker, N. & Clevers, H. Catenins, Wnt signaling and cancer. Bioessays 22, 961–965 (2000).
Hovanes, K. et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nature Genet. 28, 53–57 (2001).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Weinmaster, G. Notch signal transduction: a real Rip and more. Curr. Opin. Genet. Dev. 10, (2000).Authoritative summary of Notch signalling.
Davis, R. L. & Turner, D. L. Vertebrate Hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 20, 8342–9357 (2001).
Jarriault, S. et al. Signalling downstream of activated mammalian Notch. Nature 377, 355–358 (1995).
Oswald, F. et al. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell. Biol. 21, 7761–7774 (2001).
Ellisen, L. W. et al. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 (1991).Connects the Notch pathway to human cancer.
Callahan, D. & Callahan, R. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4). Oncogene 14, 1883–1890 (1997).
Capobianco, A. J., Zagouras, P., Blaumueller, C. M., Artavanis-Tsakonas, S. & Bishop, J. M. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol. 17, 6265–6273 (1997).
Jang, M. S., Zlobin, A., Kast, W. M. & Miele, L. Notch signaling as a target in multimodality cancer therapy. Curr. Opin. Mol. Ther. 2, 55–65 (2000).
Fitzgerald, K., Harrington, A. & Leder, P. Ras pathway signals are required for notch-mediated oncogenesis. Oncogene 19, 4191–4198 (2000).
Ingham, P. W. Transducing Hedgehog: the story so far. EMBO J. 17, 3505–3511 (1998).
Kalderon, D. Transducing the Hedgehog signal. Cell 103, 371–374 (2000).
Price, M. A. & Kalderon, D. Proteolysis of the Hedgehog signaling effector cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108, 823–835 (2002).
Ruiz i Altaba, A. Gli proteins and Hedgehog in signalling. Trends Genet. 15, 418–425 (1999).Connects human cancer with the Hedgehog pathway.
Cheng, S. Y. & Bishop, J. M. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18–mSin3 corepressor complex. Proc. Natl Acad. Sci. USA 99, 5442–5447 (2002).
Wang, B., Fallon, J. F. & Beachy, P. A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434 (2000).
Riefenberger, J. et al. Missense mutations in SMO in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 58, 1798–1803 (1998).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Hahn, H. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nature Med. 4, 619–622 (1998).
van Dam, H. & Castelazzi, M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene 20, 2453–2464 (2001).
Hartl, M. & Vogt, P. K. A rearranged junD transforms chicken embryo fibroblasts. Cell Growth Differ. 3, 909–918 (1992).
Vandel, L. et al. Stepwise transformation of rat embryo fibroblasts: c-Jun, JunB, or JunD can cooperate with Ras for focus formation, but a c-Jun-containing heterodimer is required for immortalization. Mol. Cell. Biol. 16, 1881–1888 (1996).Emphasizes the importance of multiple events in tumorigenesis.
Gille, H., Strahl, T. & Shaw, P. E. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Curr. Biol. 5, 1191–1200 (1995).
Davidson, B. et al. Ets-1 messenger RNA expression is a novel marker of poor survival in ovarian carcinoma. Clin. Cancer Res. 7, 551–557 (2001).
Shaywitz, A. J. & Greenberg, M. E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821–861 (1999).Excellent summary of CREB — one of the most well-studied transcription factors.
Fambrough, D., McClure, K., Kazlauskas, A. & Lander, E. S. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell 97, 727–741 (1999).
Gilliland, D. G. The diverse role of the ETS family of transcription factors in cancer. Clin. Cancer Res. 7, 451–453 (2001).
Nesbit, C. E., Tersak, J. M. & Prochownik, E. V. MYC oncogenes and human neoplastic disease. Oncogene 18, 3004–3016 (1999).
Grandori, C., Cowley, S. M., James, L. P. & Eisenman, R. N. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16, 653–699 (2000).Discoverer of MYC transcriptional activity provides up-to-date summary.
Eisenman, R. N. Deconstructing Myc. Genes Dev. 15, 2023–2030 (2001).
Wu, L. et al. The E2F1-3transcription factors are essential for cellular proliferation. Nature 414, 457–462 (2001).
Eymin, B., Gazzeri, S., Brambilla, C. & Brambilla, E. Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small-cell-lung carcinoma. Oncogene 20, 1678–1687 (2001).
Chen, X. et al. Gene expression patterns in human liver cancers. Mol. Biol. Cell. 13, 1929–1939 (2002).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).Among one of the first comprehensive gene-array reports: strengths and weaknesses discussed.
Rosenwald, A. et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 1937–1947 (2002).
Davis, R. E. & Staudt, L. M. Molecular diagnosis of lymphoid malignancies by gene expression profiling. Curr. Opin. Hematol. 9, 333–338 (2002).
Abbott, A. News Feature: On the offensive. Nature 416, 470–474 (2002).Summary of where cancer pharmacology stands now.
Malik, H. S., Eickbush, T. H. & Goldfarb, D. S. Evolutionary specialization of the nuclear targeting apparatus. Proc. Natl Acad. Sci. USA 94, 13738–13742 (1997).
Malik, S. & Roeder, R. G. Transcriptional regulation through mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25, 277–283 (2000).
Park, H. S., Lin, Q. & Hamilton, A. D. Supramolecular chemistry and self-assembly special feature: modulation of protein–protein interactions by synthetic receptors. Design of molecules that disrupt serine protease-proteinaceous inhibitor interaction. Proc. Natl Acad. Sci. USA 99, 5105–5109 (2002).
Ohkanda, J., Knowles, D. B., Blaskovich, M. A., Sebti, S. M. & Hamilton, A. D. Inhibitors of protein farnesyltransferase as novel anticancer agents. Curr. Top. Med. Chem. 2, 303–323 (2002).
Peczuh, M. W. & Hamilton, A. D. Peptide and protein recognition by designed molecules. Chem. Rev. 100, 2479–2494 (2000).
Cochran, A. G. Antagonists of protein–protein interactions. Chem. Biol. 7, R85–R94 (2000).
Eckert, D. M., Malashkevich, V. N., Hong, L. H., Carr, P. A. & Kim, P. S. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell 99, 103–115 (1999).
Berg, T. et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl Acad. Sci. USA 99, 3830–3835 (2002).First description of a small molecule that inhibits transcription-factor dimerization.
McBride, K. M., Banninger, G., McDonald, C. & Reich, N. C. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-α. EMBO J. 21, 1754–1763 (2002).
Haspel, R. L. & Darnell, J. E. Jr. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc. Natl Acad. Sci. USA 96, 10188–10193 (1999).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
FlyBase
LocusLink
<i>Saccharomyces</i> Genome Database
Glossary
- SRC-LIKE
-
The generic name for proteins that are similar to v-src, the oncogene of Rous sarcoma virus.
- SH2 DOMAIN
-
(Src homology 2 domain). A protein motif that recognizes and binds tyrosine-phosphorylated sequences, and thereby has a key role in relaying cascades of signal transduction.
- ANKYRIN REPEATS
-
Short amino-acid repeats that were first identified in the protein ankyrin, to which a number of cytoplasmic proteins bind.
- IMPORTIN(S)
-
A family of proteins (also called karyopherins) that combine with 'cargo' proteins in the cytoplasm and engage the nuclear import machinery to bring proteins into the nucleus.
- ENHANCEOSOME
-
A group of transcription factors that are bound to regulatory DNA elements that act in concert to activate gene transcription.
- REED–STERNBERG CELLS
-
Characteristic large stellate lymph-node cells that are associated with Hodgkin's disease.
- PROTO-ONCOGENES
-
Normal cellular genes that, when mutant or overactive, contribute to cancerous transformation in cells.
- REL PROTEINS
-
Family name for a group of proteins that have sequence similarity to the oncogene in the chicken virus (v-rel) that causes reticulo-endothelial tumours.
- WINGLESS
-
(Wg). The gene discovered early in Drosophila genetics that encodes a protein that is very similar to a DNA integrase named Int that is encoded by a retrovirus. The original term Int was melded with Wg to produce the current term WNT.
Rights and permissions
About this article
Cite this article
Darnell, J. Transcription factors as targets for cancer therapy. Nat Rev Cancer 2, 740–749 (2002). https://doi.org/10.1038/nrc906
Issue Date:
DOI: https://doi.org/10.1038/nrc906
This article is cited by
-
Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer
Signal Transduction and Targeted Therapy (2023)
-
The role and application of transcriptional repressors in cancer treatment
Archives of Pharmacal Research (2023)
-
Rational optimization of a transcription factor activation domain inhibitor
Nature Structural & Molecular Biology (2023)
-
Identification of excretory and secretory proteins from Haemonchus contortus inducing a Th9 immune response in goats
Veterinary Research (2022)
-
An overview of PROTACs: a promising drug discovery paradigm
Molecular Biomedicine (2022)