Key Points
-
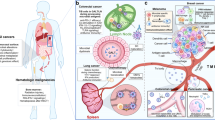
Evidence is increasing that the gut microbiota modulate the actions of chemotherapeutic drugs used in cancer and other diseases
-
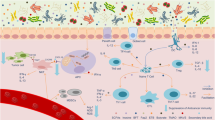
We propose the 'TIMER' mechanistic framework to explain how gut bacteria influence chemotherapy effects on the host: Translocation, Immunomodulation, Metabolism, Enzymatic degradation and Reduced diversity and ecological variation
-
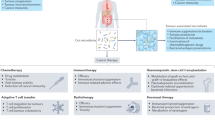
A number of tools for manipulating the gut microbiota in this context, including dietary modifications, probiotics and synthetically engineered bacteria, are in development
-
The gut microbiota will be central to the future of personalized cancer treatment strategies
Abstract
Evidence is growing that the gut microbiota modulates the host response to chemotherapeutic drugs, with three main clinical outcomes: facilitation of drug efficacy; abrogation and compromise of anticancer effects; and mediation of toxicity. The implication is that gut microbiota are critical to the development of personalized cancer treatment strategies and, therefore, a greater insight into prokaryotic co-metabolism of chemotherapeutic drugs is now required. This thinking is based on evidence from human, animal and in vitro studies that gut bacteria are intimately linked to the pharmacological effects of chemotherapies (5-fluorouracil, cyclophosphamide, irinotecan, oxaliplatin, gemcitabine, methotrexate) and novel targeted immunotherapies such as anti-PD-L1 and anti-CLTA-4 therapies. The gut microbiota modulate these agents through key mechanisms, structured as the 'TIMER' mechanistic framework: Translocation, Immunomodulation, Metabolism, Enzymatic degradation, and Reduced diversity and ecological variation. The gut microbiota can now, therefore, be targeted to improve efficacy and reduce the toxicity of current chemotherapy agents. In this Review, we outline the implications of pharmacomicrobiomics in cancer therapeutics and define how the microbiota might be modified in clinical practice to improve efficacy and reduce the toxic burden of these compounds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Wallington, M. et al. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol. 17, 1203–1216 (2016).
Mirnezami, R., Nicholson, J. & Darzi, A. Preparing for precision medicine. N. Engl. J. Med. 366, 489–491 (2012).
Marusyk, A. & Polyak, K. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta 1805, 105–117 (2010).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012).
Kantarjian, H. M., Fojo, T., Mathisen, M. & Zwelling, L. A. Cancer drugs in the United States: Justum Pretium — the just price. J. Clin. Oncol. 31, 3600–3604 (2013).
Weber, J. S. et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119, 1675–1682 (2013).
Welsh, S. J., Rizos, H., Scolyer, R. A. & Long, G. V. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur. J. Cancer 62, 76–85 (2016).
Nicholson, J. K. Global systems biology, personalized medicine and molecular epidemiology. Mol. Syst. Biol. 2, 52 (2006).
Marchesi, J. R. et al. The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339 (2016).
Holmes, E., Li, J. V., Marchesi, J. R. & Nicholson, J. K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 16, 559–564 (2012).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Nicholson, J. K., Holmes, E. & Wilson, I. D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 3, 431–438 (2005).
Li, H., He, J. & Jia, W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 12, 31–40 (2016).
Scheline, R. R. Drug metabolism by intestinal microorganisms. J. Pharm. Sci. 57, 2021–2037 (1968).
Lindenbaum, J., Tse-Eng, D., Butler, V. P. Jr & Rund, D. G. Urinary excretion of reduced metabolites of digoxin. Am. J. Med. 71, 67–74 (1981).
Lin, X. B. et al. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS ONE 9, e83644 (2014).
Jia, W., Li, H., Zhao, L. & Nicholson, J. K. Gut microbiota: a potential new territory for drug targeting. Nat. Rev. Drug Discov. 7, 123–129 (2008).
Claesson, M. J. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4586–4591 (2011).
Teillant, A., Gandra, S., Barter, D., Morgan, D. J. & Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect. Dis. 15, 1429–1437 (2015).
Samet, A. et al. Leukemia and risk of recurrent Escherichia coli bacteremia: genotyping implicates E. coli translocation from the colon to the bloodstream. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1393–1400 (2013).
Wolochow, H., Hildebrand, G. J. & Lamanna, C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J. Infect. Dis. 116, 523–528 (1966).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Schiavoni, G. et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 71, 768–778 (2011).
Viaud, S. et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 71, 661–665 (2011).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). This paper demonstrates that translocation of bacteria and stimulation of type 17 and type 1 T-helper cell responses is necessary for cyclophosphamide efficacy in tumour-bearing mice.
Daillere, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). This study reports that microbiota disruption abates tumour-associated myeloid cell responses to CpG-oligonucleotide immunotherapy and platinum chemotherapy.
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). This study shows that Bacteroidales are critical in the anti-cancer immunostimulatory effects of CTLA-4 blockade.
Wolchok, J. D. & Chan, T. A. Cancer: antitumour immunity gets a boost. Nature 515, 496–498 (2014).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015). This study establishes that Bifidobacterium improves melanoma control, facilitating anti-PD-L1 efficacy via CD8+ T-cell priming and peri-tumoral accumulation.
Berman, D. et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 10, 11 (2010).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016). This paper reports that baseline microbiota profiles enable prediction of which patients with melanoma will develop CTLA-4-blockade-induced colitis.
Abreu, M. T. et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 (2001).
Cario, E. & Podolsky, D. K. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
Frank, M. et al. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 194, 1983–1995 (2015).
Wardill, H. R. et al. Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol. Cancer Ther. 15, 1376–1386 (2016).
Paci, A. et al. Review of therapeutic drug monitoring of anticancer drugs part 1 — cytotoxics. Eur. J. Cancer 50, 2010–2019 (2014).
Villa, A. & Sonis, S. T. Mucositis: pathobiology and management. Curr. Opin. Oncol. 27, 159–164 (2015).
Touchefeu, Y. et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 40, 409–421 (2014). A systematic review summarizing evidence that the microbiota are altered in the context of mucositis, and clinical trials suggesting that probiotic use might be efficacious in this setting.
Keefe, D. M. et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109, 820–831 (2007).
Vanhoefer, U. et al. Irinotecan in the treatment of colorectal cancer: clinical overview. J. Clin. Oncol. 19, 1501–1518 (2001).
Lin, X. B. et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 7, e39764 (2012).
Gupta, E. et al. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 54, 3723–3725 (1994).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010). This study shows that diarrhoea, caused by reactivation of Irinotecan by a bacterial β -glucuronidase in the gut, is alleviated by a synthetic selective enzyme inhibitor.
Kodawara, T. et al. The inhibitory effect of ciprofloxacin on the beta-glucuronidase-mediated deconjugation of the irinotecan metabolite SN-38-G. Basic Clin. Pharmacol. Toxicol. 118, 333–337 (2016).
Kong, R. et al. Old drug new use — amoxapine and its metabolites as potent bacterial beta-glucuronidase inhibitors for alleviating cancer drug toxicity. Clin. Cancer Res. 20, 3521–3530 (2014).
Wallace, B. D. et al. Structure and inhibition of microbiome beta-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 22, 1238–1249 (2015).
Lam, W. et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl Med. 2, 45ra59 (2010).
Nakayama, H. et al. Intestinal anaerobic bacteria hydrolyse sorivudine, producing the high blood concentration of 5-(E)-(2-bromovinyl)uracil that increases the level and toxicity of 5-fluorouracil. Pharmacogenetics 7, 35–43 (1997).
Diasio, R. B. Sorivudine and 5-fluorouracil; a clinically significant drug-drug interaction due to inhibition of dihydropyrimidine dehydrogenase. Br. J. Clin. Pharmacol. 46, 1–4 (1998).
Huang, S., Li, J. Y., Wu, J., Meng, L. & Shou, C. C. Mycoplasma infections and different human carcinomas. World J. Gastroenterol. 7, 266–269 (2001).
Bronckaers, A., Balzarini, J. & Liekens, S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: implications for cancer therapy. Biochem. Pharmacol. 76, 188–197 (2008).
Vande Voorde, J. et al. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 289, 13054–13065 (2014).
Fijlstra, M. et al. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support. Care Cancer 23, 1513–1522 (2015).
Montassier, E. et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb. Ecol. 67, 690–699 (2014).
van Vliet, M. J. et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 49, 262–270 (2009).
Montassier, E. et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 42, 515–528 (2015).
Rooks, M. G. et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 8, 1403–1417 (2014).
Coley, W. B. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 3, 1–48 (1910).
Lamm, D. et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J. Urol. 191, 20–27 (2014).
Nicholson, J. K. et al. Metabolic phenotyping in clinical and surgical environments. Nature 491, 384–392 (2012).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2015).
Montassier, E. et al. Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 8, 49 (2016).
Galloway-Pena, J. R. et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 122, 2186–2196 (2016).
O'Keefe, S. J. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6, 6342 (2015).
Faber, J. et al. Bacterial translocation is reduced by a specific nutritional combination in mice with chemotherapy-induced neutropenia. J. Nutr. 141, 1292–1298 (2011).
Brandhorst, S. & Longo, V. D. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 207, 241–266 (2016).
Lee, C. et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl Med. 4, 124ra27 (2012).
Lee, J. D. et al. Synergic chemoprevention with dietary carbohydrate restriction and supplementation of AMPK-activating phytochemicals: the role of SIRT1. Eur. J. Cancer Prev. 25, 54–64 (2016).
Narta, U. K., Kanwar, S. S. & Azmi, W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit. Rev. Oncol. Hematol. 61, 208–221 (2007).
Jeon, H. et al. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget 7, 67223–67234 (2016).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Fu, Y. M., Yu, Z. X., Ferrans, V. J. & Meadows, G. G. Tyrosine and phenylalanine restriction induces G0/G1 cell cycle arrest in murine melanoma in vitro and in vivo. Nutr. Cancer 29, 104–113 (1997).
Champ, C. E. et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neurooncol. 117, 125–131 (2014).
Withers, S. S. et al. Fasting reduces the incidence of delayed-type vomiting associated with doxorubicin treatment in dogs with lymphoma. Transl Oncol. 7, 377–383 (2014).
Huisman, S. A. et al. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in Apc15lox mutant mice. Cell Cycle 14, 2333–2339 (2015).
Yun, T. K. Panax ginseng — a non-organ-specific cancer preventive? Lancet Oncol. 2, 49–55 (2001).
Wang, C. Z. et al. Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients 7, 799–814 (2015).
Wang, C. Z. et al. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother. Pharmacol. 60, 69–79 (2007).
Li, X. L. et al. Panaxadiol, a purified ginseng component, enhances the anti-cancer effects of 5-fluorouracil in human colorectal cancer cells. Cancer Chemother. Pharmacol. 64, 1097–1104 (2009).
Selma, M. V., Beltran, D., Garcia-Villalba, R., Espin, J. C. & Tomas-Barberan, F. A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 5, 1779–1784 (2014).
Gonzalez-Sarrias, A. et al. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 53, 853–864 (2014).
Gonzalez-Sarrias, A., Tome-Carneiro, J., Bellesia, A., Tomas-Barberan, F. A. & Espin, J. C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 6, 1460–1469 (2015).
Tang, Q. et al. Dietary squid ink polysaccharides ameliorated the intestinal microflora dysfunction in mice undergoing chemotherapy. Food Funct. 5, 2529–2535 (2014).
Redman, M. G., Ward, E. J. & Phillips, R. S. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann. Oncol. 25, 1919–1929 (2014).
Bowen, J. M. et al. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol. Ther. 6, 1449–1454 (2007).
Mauger, C. A., Butler, R. N., Geier, M. S., Tooley, K. L. & Howarth, G. S. Probiotic effects on 5-fluorouracil-induced mucositis assessed by the sucrose breath test in rats. Dig. Dis. Sci. 52, 612–619 (2007).
Yeung, C. Y. et al. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS ONE 10, e0138746 (2015).
Osterlund, P. et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br. J. Cancer 97, 1028–1034 (2007).
Motoori, M. et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 36, 93–99 (2017).
Wada, M. et al. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support. Care Cancer 18, 751–759 (2010).
Mego, M. et al. Prevention of febrile neutropenia in cancer patients by probiotic strain Enterococcus faecium M-74. Phase II study. Support. Care Cancer 14, 285–290 (2006).
Taper, H. S. & Roberfroid, M. B. Possible adjuvant cancer therapy by two prebiotics — inulin or oligofructose. In Vivo 19, 201–204 (2005).
Taper, H. S. & Roberfroid, M. B. Nontoxic potentiation of cancer chemotherapy by dietary oligofructose or inulin. Nutr. Cancer 38, 1–5 (2000).
Schoener, C. A., Carillo-Conde, B., Hutson, H. N. & Peppas, N. A. An inulin and doxorubicin conjugate for improving cancer therapy. J. Drug Deliv. Sci. Technol. 23, 111–118 (2013).
Kim, J. S. et al. Excess risk of Clostridium difficile infection in ovarian cancer is related to exposure to broad-spectrum antibiotics. Support. Care Cancer 21, 3103–3107 (2013).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Chung, Y. H. & Kim, D. Enhanced TLR4 expression on colon cancer cells after chemotherapy promotes cell survival and epithelial-mesenchymal transition through phosphorylation of GSK3beta. Anticancer Res. 36, 3383–3394 (2016).
Borody, T. J. & Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 9, 88–96 (2011).
Kelly, C. P. Fecal microbiota transplantation — an old therapy comes of age. N. Engl. J. Med. 368, 474–475 (2013).
Ratner, M. Seres's pioneering microbiome drug fails mid-stage trial. Nat. Biotechnol. 34, 1004–1005 (2016).
Smith, M. B., Kelly, C. & Alm, E. J. Policy: How to regulate faecal transplants. Nature 506, 290–291 (2014).
Yuvaraj, S. et al. E. coli-produced BMP-2 as a chemopreventive strategy for colon cancer: a proof-of-concept study. Gastroenterol. Res. Pract. 2012, 895462 (2012).
Din, M. O. et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85 (2016). This paper describes how synthetically engineered E. coli deliver a genetically encoded anti-neoplastic cargo in a pulsatile fashion to limit tumour activity.
Kurita, A. et al. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother. Pharmacol. 67, 201–213 (2011).
Author information
Authors and Affiliations
Contributions
J.L.A., I.D.W. and J.M.K. researched data for the article. All authors contributed equally to discussion of content, writing and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.K.N. is a nonexecutive director for Metabometrix and consultant for Waters Corporation and Nestle Research Centre. The other authors declare no competing interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Alexander, J., Wilson, I., Teare, J. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14, 356–365 (2017). https://doi.org/10.1038/nrgastro.2017.20
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.20
This article is cited by
-
Bacterial DnaK reduces the activity of anti-cancer drugs cisplatin and 5FU
Journal of Translational Medicine (2024)
-
Multi-omics analysis of the gut microbiome and metabolites associated with the psychoneurological symptom cluster in children with cancer receiving chemotherapy
Journal of Translational Medicine (2024)
-
Utilization of the microbiome in personalized medicine
Nature Reviews Microbiology (2024)
-
Role of the Microbiome in the Diagnosis and Management of Gastroesophageal Cancers
Journal of Gastrointestinal Cancer (2024)
-
Evaluation of the Treatment with Akkermansia muciniphila BAA-835 of Chemotherapy-induced Mucositis in Mice
Probiotics and Antimicrobial Proteins (2024)