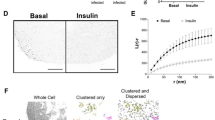
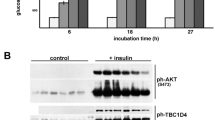
Key Points
-
Following a meal, insulin secretion from the pancreas facilitates the removal of glucose from the bloodstream. One of the main events in this regulatory process is the stimulation of glucose entry into muscle and fat cells. This is mediated by the translocation of the facilitative glucose transporter GLUT4 from an intracellular store to the cell surface.
-
The insulin-responsive GLUT4 is retained intracellularly in muscle and fat cells. In response to insulin, GLUT4 is translocated to the cell surface, where it facilitates the uptake of glucose into these insulin-responsive tissues. Understanding the transport itinerary of GLUT4 in these cells, both in the absence and presence of insulin, has proved to be a very challenging cell-biology problem.
-
A model that we believe fits all of the available data available for GLUT4 transport at present is described. This model involves the intracellular cycling of GLUT4 between the trans-Golgi network, the endosomal system and the plasma membrane. The effect of insulin on this model is discussed in the article. Other potential models are also discussed.
-
It is likely that the delivery of GLUT4 to the cell surface of fat and muscle cells is insufficient to account for the increase in glucose uptake into these cells, and therefore other layers of regulation, such as enhancement of transporter activity, might also be regulated by insulin.
-
To understand how insulin leads to the translocation of GLUT4 to the cell surface of insulin-sensitive cells, it is necessary to identify a link between the insulin-signalling pathways and GLUT4 transport machinery.
Abstract
In muscle and fat cells, insulin stimulates the delivery of the glucose transporter GLUT4 from an intracellular location to the cell surface, where it facilitates the reduction of plasma glucose levels. Understanding the molecular mechanisms that mediate this translocation event involves integrating our knowledge of two fundamental processes — the signal transduction pathways that are triggered when insulin binds to its receptor and the membrane transport events that need to be modified to divert GLUT4 from intracellular storage to an active plasma membrane shuttle service.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shepherd, P. R. & Kahn, B. B. Glucose transporters and insulin action — implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341, 248–257 (1999).
Suzuki, K. & Kono, T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc. Natl Acad. Sci. USA 77, 2542–2545 (1980).
Cushman, S. W. & Wardzala, L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 255, 4758–4762 (1980).
Stenbit, A. E. et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nature Med. 3, 1096–1101 (1997).
Brozinick, J. T. Jr et al. GLUT4 overexpression in db/db mice dose-dependently ameliorates diabetes but is not a lifelong cure. Diabetes 50, 593–600 (2001).
Holman, G. D., Leggio, L. L. & Cushman, S. W. Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 269, 17516–17524 (1994).This study used mathematical analysis to show that the intracellular transport of GLUT4 could not be explained by a simple two-compartment model, which indicates that GLUT4 is partitioned within the cell among at least two separate compartments.
Tanner, L. I. & Lienhard, G. E. Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J. Biol. Chem. 262, 8975–8980 (1987).
Albiston, A. L. et al. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. J. Biol. Chem. 13, 13 (2001).
Ross, S. A. et al. Characterization of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes. J. Biol. Chem. 271, 3328–3332 (1996).
Palacios, S. et al. Recycling of the insulin-sensitive glucose transporter GLUT4. Access of surface internalized GLUT4 molecules to the perinuclear storage compartment is mediated by the Phe5–Gln6–Gln7–Ile8 motif. J. Biol. Chem. 276, 3371–3383 (2001).
Martin, S. et al. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 134, 625–635 (1996).Using a chemical technique to ablate endosomes, this paper was one of the first to show that a significant pool of GLUT4 is excluded from endosomes and that, together with the v-SNARE VAMP2, this might demarcate a separate secretory compartment.
Martin, L. B. et al. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 273, 1444–1452 (1998).
Lampson, M. A. et al. Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell 12, 3489–3501 (2001).Using quantitative immunofluorescence-microscopy techniques, this study provides evidence that GLUT4 is retained in recycling endosomes in CHO cells, and moves to the cell surface in transport vesicles that are distinct from those that contain the TfR.
Lim, S. N. et al. Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol. Biol. Cell 12, 981–995 (2001).In vitro reconstitution experiments were used to show that GLUT4 and the TfR are sorted into different endosomally derived vesicles in CHO cells.
Slot, J. W. et al. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc. Natl Acad. Sci. USA 88, 7815–7819 (1991).
Slot, J. W. et al. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113, 123–135 (1991).This was the first immunocytochemical analysis of GLUT4 in insulin-sensitive cells and showed that insulin caused a striking redistribution of GLUT4 from intracellular tubulo–vesicular elements to the plasma membrane.
Slot, J. W. et al. Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J. Cell Biol. 137, 1–12 (1997).
Martin, S. et al. Biogenesis of insulin-responsive GLUT4 vesicles is independent of brefeldin A-sensitive trafficking. Traffic 1, 652–660 (2000).
Shewan, A. M. et al. The cytosolic C-terminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem. J. 350, 99–107 (2000).
Molloy, S. S. et al. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9, 28–35 (1999).
Xiang, Y. et al. The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol. Biol. Cell 11, 1257–1273 (2000).
Wan, L. et al. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94, 205–216 (1998).
Mallard, F. et al. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973–990 (1998).
Barr, V. A. et al. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology 138, 4463–4472 (1997).
Bogan, J. S. & Lodish, H. F. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J. Cell Biol. 146, 609–620 (1999).
Millar, C. A. et al. Adipsin and the glucose transporter GLUT4 traffic to the cell surface via independent pathways in adipocytes. Traffic 1, 141–151 (2000).
Robinson, L. J. & James, D. E. Insulin-regulated sorting of glucose transporters in 3T3-L1 adipocytes. Am. J. Physiol. 263, E383–E393 (1992).
Robinson, M. S. & Bonifacino, J. S. Adaptor-related proteins. Curr. Opin. Cell Biol. 13, 444–453 (2001).
Hashiramoto, M. & James, D. E. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol. Cell. Biol. 20, 416–427 (2000).
Ramm, G. et al. Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes. Mol. Biol. Cell 11, 4079–4091 (2000).
Kandror, K. V. & Pilch, P. F. Compartmentalization of protein traffic in insulin-sensitive cells. Am. J. Physiol. 271, E1–E14 (1996).
Roberg, K. J., Rowley, N. & Kaiser, C. A. Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J. Cell Biol. 137, 1469–1482 (1997).
Hein, C. & Andre, B. A C-terminal di-leucine motif and nearby sequences are required for NH4+-induced inactivation and degradation of the general amino acid permease, Gap1p, of Saccharomyces cerevisiae. Mol. Microbiol. 24, 607–616 (1997).
Giorgino, F. et al. The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc. Natl Acad. Sci. USA 97, 1125–1130 (2000).
Piper, R. C. & Luzio, J. P. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2, 612–621 (2001).
Sargeant, R. J. & Paquet, M. R. Effect of insulin on the rates of synthesis and degradation of GLUT1 and GLUT4 glucose transporters in 3T3-L1 adipocytes. Biochem. J. 290, 913–919 (1993).
Ploug, T. et al. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 142, 1429–1446 (1998).This study provided the first quantitative analysis of the distribution of GLUT4 in skeletal muscle after activation by either insulin, exercise or exercise plus insulin.
Millar, C. A. et al. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol. Biol. Cell 10, 3675–3688 (1999).This paper shows that in 3T3-L1 adipocytes, GLUT4 movement to the cell surface can be triggered from different intracellular pools.
Brozinick, J. T. Jr & Birnbaum, M. J. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J. Biol. Chem. 273, 14679–14682 (1998).
Foran, P. G. et al. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J. Biol. Chem. 274, 28087–28095 (1999).
Somwar, R. et al. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem. J. 359, 639–649 (2001).
Karnielli, E. et al. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. J. Biol. Chem. 256, 4772–4777 (1981).
Molero, J. C. et al. Nocodazole inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes via a microtubule-independent mechanism. J. Biol. Chem. 276, 43829–43835 (2001).
Hausdorff, S. F. et al. Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes. Dissociation of insulin-stimulated glucose uptake and GLUT4 translocation. J. Biol. Chem. 274, 24677–24684 (1999).
Somwar, R. et al. Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J. Biol. Chem. 276, 46079–46087 (2001).
Sweeney, G. et al. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J. Biol. Chem. 274, 10071–10078 (1999).
Sweeney, G. et al. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in L6 rat skeletal muscle cells. Endocrinology 142, 4806–4812 (2001).
Joost, H. G. et al. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J. Biol. Chem. 261, 10033–10036 (1986).
Lawrence, J. C. et al. GLUT4 facilitates insulin stimulation and cAMP-mediated inhibition of glucose transport. Proc. Natl Acad. Sci. USA 89, 3493–3497 (1992).
Clancy, B. M. et al. Protein synthesis inhibitors activate glucose transport without increasing plasma membrane glucose transporters in 3T3-L1 adipocytes. J. Biol. Chem. 266, 10122–10130 (1991).
James, D. E., Hiken, J. & Lawrence, J. C. Isoproterenol stimulates phosphorylation of the insulin-regulatable glucose transporter in rat adipocytes. Proc. Natl Acad. Sci. USA 86, 8368–8372 (1989).
Piper, R. C. et al. GLUT4 phosphorylation and inhibition of glucose transport by dibutyryl cAMP. J. Biol. Chem. 268, 16557–16563 (1993).
Zottola, R. J. et al. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry 34, 9734–9747 (1995).
Robinson, L. J. et al. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP, insulin and GTPγS and localization of GLUT4 to clathrin lattices. J. Cell Biol. 117, 1181–1196 (1992).
Ros-Baro, A. et al. Lipid rafts are required for GLUT4 internalization in adipose cells. Proc. Natl Acad. Sci. USA 98, 12050–12055 (2001).
Inoue, G., Cheatham, B. & Kahn, C. R. Development of an in vitro reconstitution assay for glucose transporter 4 translocation. Proc. Natl Acad. Sci. USA 96, 14919–14924 (1999).This paper was the first to show that it is feasible to reconstitute the docking and fusion of intracellular GLUT4 vesicles with the plasma membrane in vitro.
Sanchez, P. et al. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol. 18, 3069–3080 (1998).
Min, J. et al. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 3, 751–760 (1999).
Brooks, C. C. et al. Pantophysin is a phosphoprotein component of adipocyte transport vesicles and associates with GLUT4-containing vesicles. J. Biol. Chem. 275, 2029–2036 (2000).
Foster, L. J. et al. A functional role for VAP-33 in insulin-stimulated GLUT4 traffic. Traffic 1, 512–521 (2000).
Shibata, H., Omata, W. & Kojima, I. Insulin stimulates guanine nucleotide exchange on Rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J. Biol. Chem. 272, 14542–14546 (1997).
Li, L. et al. Direct interaction of Rab4 with syntaxin 4. J. Biol. Chem. 276, 5265–5273 (2001).
Braiman, L. et al. Activation of protein kinase Cζ induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol. Cell. Biol. 21, 7852–7861 (2001).
Martin, S. S. et al. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 271, 17605–17608 (1996).
Kohn, A. D. et al. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372–31378 (1996).
Chiang, S. H. et al. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948 (2001).
Tong, P. et al. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Invest. 108, 371–381 (2001).
Emoto, M., Langille, S. E. & Czech, M. P. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J. Biol. Chem. 276, 10677–10682 (2001).
Kanzaki, M. & Pessin, J. E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276, 42436–42444 (2001).
Kanzaki, M. et al. Insulin stimulates actin comet tails on intracellular GLUT4-containing compartments in differentiated 3T3L1 adipocytes. J. Biol. Chem. 276, 49331–49336 (2001).
Harris, M. I. et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21, 518–524 (1998).
Watson, R. T. et al. Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154, 829–840 (2001).
Foster, L. J. & Klip, A. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell Physiol. 279, C877–C890 (2000).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nature Rev. Mol. Cell Biol. 2, 107–117 (2001).
Kuboshima, S. et al. Hyperosmotic stimuli induces recruitment of aquaporin-1 to plasma membrane in cultured rat peritoneal mesothelial cells. Adv. Perit. Dial. 17, 47–52 (2001).
Marinelli, R. A. et al. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane. Evidence for a secretin-induced vesicular translocation of aquaporin-1. J. Biol. Chem. 272, 12984–12988 (1997).
Kamsteeg, E. J. et al. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J. Cell Biol. 151, 919–930 (2000).
Gustafson, C. E. et al. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am. J. Physiol. Renal Physiol. 278, F317–F326 (2000).
Mandon, B. et al. Syntaxin-4 is localized to the apical plasma membrane of rat renal collecting duct cells: possible role in aquaporin-2 trafficking. J. Clin. Invest. 98, 906–913 (1996).
Valenti, G. et al. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J. Cell Sci. 113, 1985–1992 (2000).
Klussmann, E. et al. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 274, 4934–4938 (1999).
Katsura, T. et al. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am. J. Physiol. 272, F817–F822 (1997).
Valenti, G. et al. A heterotrimeric G protein of the Gi family is required for cAMP- triggered trafficking of aquaporin 2 in kidney epithelial cells. J. Biol. Chem. 273, 22627–22634 (1998).
Mulders, S. M. et al. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Invest. 102, 57–66 (1998).
Snyder, P. M. Liddle's syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na+ channel to the cell surface. J. Clin. Invest. 105, 45–53 (2000).
Loffing, J. et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am. J. Physiol. Renal. Physiol. 280, F675–F682 (2001).
Djelidi, S. et al. Basolateral translocation by vasopressin of the aldosterone-induced pool of latent Na-K-ATPases is accompanied by α1 subunit dephosphorylation: study in a new aldosterone-sensitive rat cortical collecting duct cell line. J. Am. Soc. Nephrol. 12, 1805–1818 (2001).
Juel, C., Nielsen, J. J. & Bangsbo, J. Exercise-induced translocation of Na+-K+ pump subunits to the plasma membrane in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1107–R1110 (2000).
Lavoie, L. et al. Insulin-induced translocation of Na+-K+-ATPase subunits to the plasma membrane is muscle fiber type specific. Am. J. Physiol. 270, C1421–C1429 (1996).
Omatsu-Kanbe, M. & Kitasato, H. Insulin stimulates the translocation of Na+/K+-dependent ATPase molecules from intracellular stores to the plasma membrane in frog skeletal muscle. Biochem. J. 272, 727–733 (1990).
Aledo, J. C. & Hundal, H. S. Sedimentation and immunological analyses of GLUT4 and α2-Na,K-ATPase subunit-containing vesicles from rat skeletal muscle: evidence for segregation. FEBS Lett. 376, 211–215 (1995).
Janecki, A. J. et al. Basic fibroblast growth factor stimulates surface expression and activity of Na+/H+ exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 8133–8142 (2000).
D'Souza, S. et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J. Biol. Chem. 273, 2035–2043 (1998).
Boels, K. et al. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca2+-permeable channel GRC. J. Cell Sci. 114, 3599–3606 (2001).
Kanzaki, M. et al. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-1. Nature Cell Biol. 1, 165–170 (1999).
Passafaro, M. et al. N-type Ca2+ channels are present in secretory granules and are transiently translocated to the plasma membrane during regulated exocytosis. J. Biol. Chem. 271, 30096–30104 (1996).
Shintani, Y. & Marunaka, Y. Regulation of chloride channel trafficking by cyclic AMP via protein kinase A-independent pathway in A6 renal epithelial cells. Biochem. Biophys. Res. Commun. 223, 234–239 (1996).
Agnew, B. J. et al. Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J. Cell Sci. 112, 2639–2646 (1999).
Petris, M. J. & Mercer, J. F. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum. Mol. Genet. 8, 2107–2115 (1999).
Yamaguchi, Y. et al. Biochemical characterization and intracellular localization of the Menkes disease protein. Proc. Natl Acad. Sci. USA 93, 14030–14035 (1996).
Quick, M. W. et al. Second messengers, trafficking-related proteins, and amino acid residues that contribute to the functional regulation of the rat brain GABA transporter GAT1. J. Neurosci. 17, 2967–2979 (1997).
Sims, K. D., Straff, D. J. & Robinson, M. B. Platelet-derived growth factor rapidly increases activity and cell surface expression of the EAAC1 subtype of glutamate transporter through activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 5228–5237 (2000).
Chklovskaia, E. et al. Mechanism of flt3 ligand expression in bone marrow failure: translocation from intracellular stores to the surface of T lymphocytes after chemotherapy-induced suppression of hematopoiesis. Blood 93, 2595–2604 (1999).
Chklovskaia, E. et al. Cell-surface trafficking and release of flt3 ligand from T lymphocytes is induced by common cytokine receptor γ-chain signaling and inhibited by cyclosporin A. Blood 97, 1027–1034 (2001).
Shuster, S. J. et al. Stimulus-dependent translocation of κ opioid receptors to the plasma membrane. J. Neurosci. 19, 2658–2664 (1999).
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- FACILITATIVE SUGAR TRANSPORTER
-
A polytopic membrane protein that transports sugars down a concentration gradient in an energy-independent manner.
- TYPE II DIABETES
-
Also known as non-insulin-dependent diabetes or maturity onset diabetes.
- DB/DB MOUSE
-
A genetic mouse model of type II diabetes and obesity. The defect has been mapped to the gene for the leptin receptor.
- EVANESCENCE WAVE MICROSCOPY
-
A technique in which only fluorophores within a 100–220 nm field above a glass coverslip are excited, which allows localization of molecules very close to the cell surface.
- ATRIAL CARDIOMYOCYTE
-
A heart muscle cell.
- AP-1
-
(Adaptor protein complex 1). Adaptor proteins link cargo molecules on membranes with coat proteins such as clathrin. Several classes of adaptor proteins have been identified and shown to be involved in different transport steps. AP-1 is thought to regulate transport from the trans-Golgi network to endosomes.
- SNARE
-
(soluble N-ethylmaleimide sensitive factor attachment protein receptor). A family of membrane-tethered coiled-coil proteins that regulate fusion reactions and target specificity in the vacuolar system. They can be divided into v-SNAREs (vesicle) and t-SNAREs (target) on the basis of their localization, or into Q-SNAREs and R-SNAREs on the basis of a highly conserved amino acid.
- CONVERTASE
-
An enzyme that is responsible for protein activation through proteolytic activity.
- COAT-ASSOCIATED PROTEIN
-
A protein that links cargo molecules to vesicle coats.
- ARNO
-
(ARF nucleotide binding-site opener). This activates ADP-ribosylating factors (ARFs), which are known to have a role in protein sorting and vesicle budding.
- γ-EAR-CONTAINING
-
This represents a protein domain within the γ-subunit of coat adaptor proteins.
- CD-MPR
-
(Cation-dependent mannose-6-phosphate receptor). This protein shuttles between the trans-Golgi network and endosomes.
- GTPγS
-
A non-hydrolysable analogue of GTP.
- BREFELDIN A
-
A fungal metabolite that affects membrane transport and the structure of the Golgi apparatus.
Rights and permissions
About this article
Cite this article
Bryant, N., Govers, R. & James, D. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3, 267–277 (2002). https://doi.org/10.1038/nrm782
Issue Date:
DOI: https://doi.org/10.1038/nrm782
This article is cited by
-
Combined transcriptome and metabolome analysis reveals breed-specific regulatory mechanisms in Dorper and Tan sheep
BMC Genomics (2024)
-
Environmental exposure to lead and cadmium are associated with triglyceride glucose index
Scientific Reports (2024)
-
Tumor suppressor let-7 acts as a key regulator for pluripotency gene expression in Muse cells
Cellular and Molecular Life Sciences (2024)
-
Monoamine oxidase mediated oxidative stress: a potential molecular and biochemical crux in the pathogenesis of obesity
Molecular Biology Reports (2024)
-
Endothelial ERα promotes glucose tolerance by enhancing endothelial insulin transport to skeletal muscle
Nature Communications (2023)