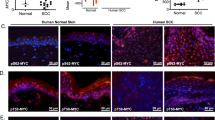
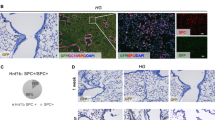
Abstract
Inositol hexakisphosphate kinase 2 (IP6K2), a member of the inositol hexakisphosphate kinase family, functions as a growth suppressive and apoptosis-enhancing kinase during cell stress. We created mice with a targeted deletion of IP6K2; these mice display normal embryogenesis, development, growth and fertility. Chronic exposure to the carcinogen 4-nitroquinoline 1-oxide (4-NQO, a UV-mimetic compound) in drinking water resulted in fourfold increased incidence of invasive squamous cell carcinoma (SCC) formation in the oral cavity and esophagus of the knockout (KO) mice compared to the wild-type (WT) littermates. Paradoxically, KO mice displayed relative resistance to ionizing radiation and exhibit enhanced survival following 8–10 Gy total body irradiation. Primary KO fibroblasts displayed resistance to antiproliferative effects of interferon-β and increased colony forming units following ionizing radiation. Radioresistance of KO fibroblasts was associated with accelerated DNA repair measured by comet assay. Direct microinjection of 5-PP-Ins(1,2,3,4,6)P5 (the enzymatic product of IP6K2), but not InsP6 (the substrate of IP6K2) induced cell death in SCC22A squamous carcinoma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
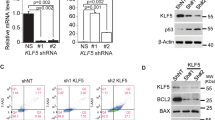
Abbreviations
- IP6K2:
-
inositol hexakisphosphate kinase 2
- 4-NQO:
-
4-nitroquinoline 1-oxide
- IFN:
-
interferon
- HNSCC:
-
head and neck squamous cell carcinoma
- NHEJ:
-
nonhomologous end joining
- PBS:
-
phosphate-buffered saline
References
Bhandari R, Juluri KR, Resnick AC, Snyder SH . (2008). Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc Natl Acad Sci USA 105: 2349–2353.
Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ et al. (2007). Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci USA 104: 15305–15310.
Byrum J, Jordan S, Safrany ST, Rodgers W . (2004). Visualization of inositol phosphate-dependent mobility of Ku: depletion of the DNA-PK cofactor InsP6 inhibits Ku mobility. Nucleic Acids Res 32: 2776–2784.
Chakraborty SB, Dasgupta S, Roy A, Sengupta A, Ray B, Roychoudhury S et al. (2003). Differential deletions in 3p are associated with the development of head and neck squamous cell carcinoma in Indian patients. Cancer Genet Cytogenet 146: 130–138.
Cheung JC, Salerno B, Hanakahi LA . (2008). Evidence for an inositol hexakisphosphate-dependent role for Ku in mammalian nonhomologous end joining that is independent of its role in the DNA-dependent protein kinase. Nucleic Acids Res, 2008 36: 5713–5726.
Fridy PC, Otto JC, Dollins DE, York JD . (2007). Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J Biol Chem 282: 30754–30762.
Hanakahi LA, Bartlet-Jones M, Chappell C, Pappin D, West SC . (2000). Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell 102: 721–729.
Hawkins BL, Heniford BW, Ackermann DM, Leonberger M, Martinez SA, Hendler FJ . (1994). 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck 16: 424–432.
Hecht JT, Hogue D, Strong LC, Hansen MF, Blanton SH, Wagner M . (1995). Hereditary multiple exostosis and chondrosarcoma: linkage to chromosome II and loss of heterozygosity for EXT-linked markers on chromosomes II and 8. Am J Hum Genet 56: 1125–1131.
Hoornaert I, Marynen P, Goris J, Sciot R, Baens M . (2003). MAPK phosphatase DUSP16/MKP-7, a candidate tumor suppressor for chromosome region 12p12-13, reduces BCR-ABL-induced transformation. Oncogene 22: 7728–7736.
Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K et al. (2007). Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science 318: 1299–1302.
Kawata T, Ito H, Saito M, Uno T, Okayasu R, Liu C et al. (2005). Caffeine sensitizes nondividing human fibroblasts to x rays by inducing a high frequency of misrepair. Radiat Res 164: 509–513.
Kendall J, Liu Q, Bakleh A, Krasnitz A, Nguyen KC, Lakshmi B et al. (2007). Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci USA 104: 16663–16668.
Kibel AS, Huagen J, Guo C, Isaacs WB, Yan Y, Pienta KJ et al. (2004). Expression mapping at 12p12-13 in advanced prostate carcinoma. Int J Cancer 109: 668–672.
Kok K, Osinga J, Carritt B, Davis MB, van der Hout AH, van der Veen AY et al. (1987). Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature 330: 578–581.
Li X, Lee NK, Ye YW, Waber PG, Schweitzer C, Cheng QC et al. (1994). Allelic loss at chromosomes 3p, 8p, 13q, and 17p associated with poor prognosis in head and neck cancer. J Natl Cancer Inst 86: 1524–1529.
Lu B, Xu J, Lai M, Zhang H, Chen J . (2006). A transcriptome anatomy of human colorectal cancers. BMC Cancer 6: 40.
Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K et al. (2003). Inositol pyrophosphates mediate chemotaxis in dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 114: 559–572.
Ma Y, Lieber MR . (2002). Binding of inositol hexakisphosphate (IP6) to Ku but not to DNA-PKcs. J Biol Chem 30: 30.
Maestro R, Gasparotto D, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M . (1993). Three discrete regions of deletion at 3p in head and neck cancers. Cancer Res 53: 5775–5779.
Magnusdottir E, Kalachikov S, Mizukoshi K, Savitsky D, Ishida-Yamamoto A, Panteleyev AA et al. (2007). Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci USA 104: 14988–14993.
Morrison BH, Bauer JA, Hu J, Grane RW, Ozdemir A, Chawla-Sarkar M et al. (2002). Inositol hexakisphosphate kinase 2 sensitizes ovarian carcinoma cells to multiple cancer therapeutics. Oncogene 21: 1882–1889.
Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ . (2001). Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-beta in ovarian carcinoma cells. J Biol Chem 276: 24965–24970.
Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE et al. (2007). A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316: 106–109.
Nagata E, Luo HR, Saiardi A, Bae BI, Suzuki N, Snyder SH . (2005). Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem 280: 1634–1640.
Panigrahi GB, Walker IG . (1990). The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3′-5′ exonuclease action of T4 DNA polymerase. Biochemistry 29: 2122–2126.
Pogach MS, Cao Y, Millien G, Ramirez MI, Williams MC . (2007). Key developmental regulators change during hyperoxia-induced injury and recovery in adult mouse lung. J Cell Biochem 100: 1415–1429.
Raskind WH, Conrad EU, Chansky H, Matsushita M . (1995). Loss of heterozygosity in chondrosarcomas for markers linked to hereditary multiple exostoses loci on chromosomes 8 and 11. Am J Hum Genet 56: 1132–1139.
Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH . (2004). Phosphorylation of proteins by inositol pyrophosphates. Science 306: 2101–2105.
Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH . (2005). Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc Natl Acad Sci USA 102: 1911–1914.
Saiardi A, Sciambi C, McCaffery JM, Wendland B, Snyder SH . (2002). Inositol pyrophosphates regulate endocytic trafficking. Proc Natl Acad Sci USA 99: 14206–14211.
Shears SB . (2004). How versatile are inositol phosphate kinases? Biochem J 377: 265–280.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112.
Teng CH, Huang WN, Meng TC . (2007). Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. J Biol Chem 282: 28395–28407.
Wojewodzka M, Buraczewska I, Kruszewski M . (2002). A modified neutral comet assay: elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat Res 518: 9–20.
York SJ, Armbruster BN, Greenwell P, Petes TD, York JD . (2005). Inositol diphosphate signaling regulates telomere length. J Biol Chem 280: 4264–4269.
Zhang H, Thompson J, Prestwich GD . (2009). A scalable synthesis of the IP(7) Isomer, 5-PP-Ins(1,2,3,4,6)P(5). Org Lett 11: 1551–1554.
Acknowledgements
We thank Philip Sanford, University of Cincinnati, for design of targeting vector and creation of IHPK2 knockout mice. Donna Kusewitt, Ohio State University, Director, Mouse Phenotyping Shared Resource, kusewitt.1@osu.edu, conducted the phenotyping studies. Judy Drazba, Cleveland Clinic Imaging Core, performed the time-lapse photography of microinjected cells. Dr Yong Xu, Dr Honglu Zhang and Dr Jianxing Zhang performed the synthesis of 5-PP-Ins(1,2,3,4,6)P5. These studies were supported by grants from NIH/NCI (CA095020) to DJL and from NIH (NS29632) to GDP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Morrison, B., Haney, R., Lamarre, E. et al. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene 28, 2383–2392 (2009). https://doi.org/10.1038/onc.2009.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.113
Keywords
This article is cited by
-
Inositol hexakisphosphate kinase 2 promotes cell death of anterior horn cells in the spinal cord of patients with amyotrophic lateral sclerosis
Molecular Biology Reports (2020)
-
Inositol pyrophosphates mediated the apoptosis induced by hypoxic injury in bone marrow-derived mesenchymal stem cells by autophagy
Stem Cell Research & Therapy (2019)
-
Inositol Pyrophosphates: Energetic, Omnipresent and Versatile Signalling Molecules
Journal of the Indian Institute of Science (2017)
-
Inositol Hexakisphosphate Kinase 3 Regulates Metabolism and Lifespan in Mice
Scientific Reports (2016)
-
The emerging roles of inositol pyrophosphates in eukaryotic cell physiology
Journal of Biosciences (2015)