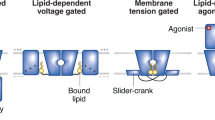
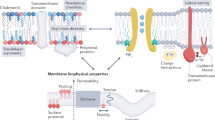
Abstract
Biologic membranes are not simply inert physical barriers, but complex and dynamic environments that affect membrane protein structure and function. Residing within these environments, ion channels control the flux of ions across the membrane through conformational changes that allow transient ion flux through a central pore. These conformational changes may be modulated by changes in transmembrane electrochemical potential, the binding of small ligands or other proteins, or changes in the local lipid environment. Ion channels play fundamental roles in cellular function and, in higher eukaryotes, are the primary means of intercellular signaling, especially between excitable cells such as neurons. The focus of this review is to examine how the composition of the bilayer affects ion channel structure and function. This is an important consideration because the bilayer composition varies greatly in different cell types and in different organellar membranes. Even within a membrane, the lipid composition differs between the inner and outer leaflets, and the composition within a given leaflet is both heterogeneous and highly dynamic. Differential packing of lipids (and proteins) leads to the formation of microdomains, and lateral diffusion of these microdomains or “lipid rafts” serve as mobile platforms for the clustering and organization of bilayer constituents including ion channels. The structure and function of these channels are sensitive to specific chemical interactions with neighboring components of the membrane and also to the biophysical properties of their membrane microenvironment (e.g., fluidity, lateral pressure profile, and bilayer thickness). As specific examples, we have focused on the K+ ion channels and the ligand-gated nicotinicoid receptors, two classes of ion channels that have been well-characterized structurally and functionally. The responsiveness of these ion channels to changes in the lipid environment illustrate how ion channels, and more generally, any membrane protein, may be regulated via cellular control of membrane composition.
Similar content being viewed by others
References
Dowhan, W. (1997) Molecular basis for membrane phospholipid diversity: Why are there so many lipids. Annu. Rev. Biochem. 66, 199–232.
White, S. H. and Wimley, W. C. (1999) Membrane protein folding and stability: Physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365.
Popot, J. L. and Engelman, D. M. (2000) Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 69, 881–922.
Grigorieff, N., Ceska, T. A., Downing, K. H., Baldwin, J. M., and Henderson, R. (1996) Electron-crystallographic refinement of the structure of bacteriorhodopsin. J. Mol. Biol. 259, 393–421.
Joshi, M. K., Dracheva, S., Mukhopadhyay, A. K., Bose, S., and Hendler, R. W. (1998) Importance of specific native lipids in controlling the photocycle of bacteriorhodopsin. Biochemistry 37, 14463–14470.
le Maire, M., Champeil, P., and Moller, J. V. (2000) Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 1508, 86–111.
Ridder, A. N., Morein, S., Stam, J. G., Kuhn, A., de Kruijff, B., and Killian, J. A. (2000) Analysis of the role of interfacial tryptophan residues in controlling the topology of membrane proteins. Biochemistry 39, 6521–6528.
Yau, W. M., Wimley, W. C., Gawrisch, K., and White, S. H. (1998) The preference of tryptophan for membrane interfaces. Biochemistry 37, 14713–14718.
Wimley, W. C., and White, S. H. (1996) Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3, 842–848.
Schiffer, M., Chang, C. H., and Stevens, F. J. (1992) The functions of tryptophan residues in membrane proteins. Protein Eng. 5, 213–214.
Rees, D. C., DeAntonio, L., and Eisenberg, D. (1989) Hydrophobic organization of membrane proteins. Science 245, 510–513.
Ubarretxena-Belandia, I., and Engelman, D. M. (2001) Helical membrane proteins: diversity of functions in the context of simple architecture. Curr. Opin. Struct. Biol. 11, 370–376.
Eilers, M., Patel, A. B., Liu, W., and Smith, S. O. (2002) Comparison of helix interactions in membrane and soluble alpha-bundle proteins. Biophys. J. 82, 2720–2736.
Choma, C., Gratkowski, H., Lear, J. D., and DeGrado, W. F. (2000) Asparagine-mediated self-association of a model transmembrane helix. Nat. Struct. Biol. 7, 161–166.
Zhou, F. X., Cocco, M. J., Russ, W. P., Brunger, A. T., and Engelman, D. M. (2000) Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat. Struct. Biol. 7, 154–160.
Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T., and MacKinnon, R. (1998) The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280, 69–77.
Zhou, Y., Morais-Cabral, J. H., Kaufman, A., and MacKinnon, R. (2001) Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 414, 43–48.
Fu, D., Libson, A., Miercke, L. J., Weitzman, C., Nollert, P., Krucinski, J., and Stroud, R. M. (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290, 481–486.
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R. (2002) X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294.
Lodish, H. F. (1988) Multi-spanning membrane proteins: How accurate are the models? Trends Biochem. Sci. 13, 332–334.
Hamasaki, N., Abe, Y., and Tanner, M. J. (2002) Flexible regions within the membrane-embedded portions of polytopic membrane proteins. Biochemistry 41, 3852–3854.
Pebay-Peyroula, E., and Rosenbusch, J. P. (2001) High-resolution structures and dynamics of membrane protein—lipid complexes: A critique. Curr. Opin. Struct. Biol. 11, 427–432.
Fyfe, P. K., McAuley, K. E., Roszak, A. W., Isaacs, N. W., Cogdell, R. J., and Jones, M. R. (2001) Probing the interface between membrane proteins and membrane lipids by X-ray crystallography. Trends Biochem. Sci. 26, 106–112.
Marsh, D. and Horvath, L. I. (1998) Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim. Biophys. Acta 1376, 267–296.
Melkonian, K. A., Ostermeyer, A. G., Chen, J. Z., Roth, M. G., and Brown, D. A. (1999) Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274, 3910–3917.
Anderson, R. G. and Jacobson, K. A. (2002) role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825.
Bogdanov, M. and Dowhan, W. (1999) Lipid-assisted protein folding. J. Biol. Chem. 274, 36827–36830.
Bogdanov, M. and Dowhan, W. (1998) Phospholipid-assisted protein folding: Phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. Embo J. 17, 5255–5264.
Freudl, R., Schwarz, H., Stierhof, Y. D., Gamon, K., Hindennach, I., and Henning, U. (1986) An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J. Biol. Chem. 261, 11355–11361.
de Cock, H., van Blokland, S., and Tommassen, J. (1996) In vitro insertion and assembly of outer membrane protein PhoE of Escherichia coli K-12 into the outer membrane. Role of Triton X-100. J. Biol. Chem. 271, 12885–12890.
Holzenburg, A., Engel, A., Kessler, R., Manz, H. J., Lustig, A., and Aebi, U. (1989) Rapid isolation of OmpF porin-LPS complexes suitable for structure-function studies. Biochemistry 28, 4187–4193.
Eisele, J. L. and Rosenbusch, J. P. (1990) In vitro folding and oligomerization of a membrane protein. Transition of bacterial porin from random coil to native conformation. J. Biol. Chem. 265, 10217–10220.
Ferguson, A. D., Welte, W., Hofmann, E., Lindner, B., Holst, O., Coulton, J. W., and Diederichs, K. (2000) A conserved structural motif for lipopolysaccharide recognition by procaryotic and eucaryotic proteins. Structure Fold Des 8, 585–592.
Hwang, P. M. and Vogel, H. J. (1998) Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 76, 235–246.
Johnson, J. E. and Cornell, R. B. (1999) Amphitropic proteins: regulation by reversible membrane interactions (review). Mol. Membr. Biol. 16, 217–235.
Fivaz, M., Abrami, L., and van der Goot, F. G. (1999) Landing on lipid rafts. Trends Cell Biol. 9, 212–213.
Schwarze, S. R. and Dowdy, S. F. (2000) In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. Sci. 21, 45–48.
Naslavsky, N., Shmeeda, H., Friedlander, G., Yanai, A., Futerman, A. H., Barenholz, Y., and Taraboulos, A. (1999) Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J. Biol. Chem. 274, 20763–20771.
Kurzchalia, T. V. and Parton, R. G. (1999) Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11, 424–431.
Mahfoud, R., Garmy, N., Maresca, M., Yahi, N., Puigserver, A., and Fantini, J. (2002) Identification of a common sphingolipid-binding domain in Alzheimer, prion, and HIV-1 proteins. J. Biol. Chem. 277, 11292–11296.
Jo, E., McLaurin, J., Yip, C. M., St George-Hyslop, P., and Fraser, P. E. (2000) α-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 275, 34328–34334.
Lehtonen, J. Y. and Kinnunen, P. K. (1997) Evidence for phospholipid microdomain formation in liquid crystalline liposomes reconstituted with Escherichia coli lactose permease. Biophys. J. 72, 1247–1257.
Polozova, A. and Litman, B. J. (2000) Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 79, 2632–2643.
Epand, R. M., Maekawa, S., Yip, C. M., and Epand, R. F. (2001) Protein-induced formation of cholesterol-rich domains. Biochemistry 40, 10514–10521
daCosta, C. J., Ogrel, A. A., McCardy, E. A., Blanton, M. P., and Baenziger, J. E. (2002) Lipid-protein interactions at the nicotinic acetylcholine receptor. A functional coupling between nicotinic receptors and phosphatidic acid- containing lipid bilayers. J. Biol. Chem. 277, 201–208.
Radhakrishnan, A. and McConnell, H. M. (2000) Chemical activity of cholesterol in membranes. Biochemistry 39, 8119–8124.
Miao, L., Nielsen, M., Thewalt, J., Ipsen, J. H., Bloom, M., Zuckermann, M. J., and Mouritsen, O. G. (2002) From lanosterol to cholesterol: structural evolution and differential effects on lipid bilayers. Biophys. J. 82, 1429–1444.
Barenholz, Y. (2002) Cholesterol and other membrane active sterols: from membrane evolution to “rafts”. Prog. Lipid Res. 41, 1–5.
Gimpl, G., Burger, K., and Fahrenholz, F. (1997) Cholesterol as modulator of receptor function. Biochemistry 36, 10959–10974.
Ding, J., Starling, A. P., East, J. M., and Lee, A. G. (1994) Binding sites for cholesterol on Ca2+-ATPase studies by using a cholesterol-containing phospholipid. Biochemistry 33, 4974–4979.
Niu, S. L., Mitchell, D. C., and Litman, B. J. (2002) Manipulation of cholesterol levels in rod disk membranes by methyl-β-cyclodextrin. Effects on receptor activation. J. Biol. Chem. 277, 20139–20145.
Booth, P. J., Templer, R. H., Meijberg, W., Allen, S. J., Curran, A. R., and Lorch, M. (2001) In vitro studies of membrane protein folding. Crit. Rev. Biochem. Mol. Biol. 36, 501–603.
Cantor, R. S. (1999) Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76, 2625–2639.
Cantor, R. S. (2002) Size distribution of barrelstave aggregates of membrane peptides: Influence of the bilayer lateral pressure profile. Biophys. J. 82, 2520–2525.
Epand, R. M. (1998) Lipid polymorphism and protein-lipid interactions. Biochim. Biophys. Acta 1376, 353–368.
Nielsen, C., Goulian, M., and Andersen, O. S. (1998) Energetics of inclusion-induced bilayer deformations. Biophys. J. 74, 1966–1983.
Curran, A. R., Templer, R. H., and Booth, P. J. (1999) Modulation of folding and assembly of the membrane protein bacteriorhodopsin by intermolecular forces within the lipid bilayer. Biochemistry 38, 9328–9336.
Spencer, R. H., Chang, G., and Rees, D. C. (1999) ‘Feeling the pressure’: Structural insights into a gated mechanosensitive channel. Curr. Opin. Struct. Biol. 9, 448–454.
Hamill, O. P., and Martinac, B. (2001) Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81, 685–740.
Patel, A. J., Lazdunski, M., and Honore, E. (2001) Lipid and mechano-gated 2P domain K+ channels. Curr. Opin. Cell Biol. 13, 422–428.
Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A., and Martinac, B. (2002) Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948.
Baenziger, J. E., Darsaut, T. E., and Morris, M. L. (1999) Internal dynamics of the nicotinic acetylcholine receptor in reconstituted membranes. Biochemistry 38, 4905–4911.
Ren, J., Lew, S., Wang, J., and London, E. (1999) Control of the transmembrane orientation and interhelical interactions within membranes by hydrophobic helix length. Biochemistry 38, 5905–5912.
Ren, J., Lew, S., Wang, Z., and London, E. (1997) Transmembrane orientation of hydrophobic alpha-helices is regulated both by the relationship of helix length to bilayer thickness and by the cholesterol concentration. Biochemistry 36, 10213–10220.
Mall, S., Broadbridge, R., Sharma, R. P., Lee, A. G., and East, J. M. (2000) Effects of aromatic residues at the ends of transmembrane α-helices on helix interactions with lipid bilayers. Biochemistry 39, 2071–2078.
Lewis, B. A., and Engelman, D. M. (1983) Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 166, 211–217.
Sankaram, M. B. and Thompson, T. E. (1990) Modulation of phospholipid acyl chain order by cholesterol. A solid- state 2H nuclear magnetic resonance study. Biochemistry 29, 10676–10684.
Pilot, J. D., East, J. M., and Lee, A. G. (2001) Effects of bilayer thickness on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry 40, 8188–8195.
Cornelius, F. (2001) Modulation of Na, K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry 40, 8842–8851.
Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655.
Lee, A. G. (1998) How lipids interact with an intrinsic membrane protein: The case of the calcium pump. Biochim. Biophys. Acta 1376, 381–390.
Caffrey, M., and Feigenson, G. W. (1981) Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry 20, 1949–1961.
Johannsson, A., Keightley, C. A., Smith, G. A., Richards, C. D., Hesketh, T. R., and Metcalfe, J. C. (1981) The effect of bilayer thickness and n-alkanes on the activity of the (Ca2++Mg2+)-dependent ATPase of sarcoplasmic reticulum. J. Biol. Chem. 256, 1643–1650.
Starling, A. P., East, J. M., and Lee, A. G. (1993) Effects of phosphatidylcholine fatty acyl chain length on calcium binding and other functions of the (Ca2+−Mg2+)-ATPase. Biochemistry 32, 1593–1600.
Starling, A. P., East, J. M., and Lee, A. G. (1995) Evidence that the effects of phospholipids on the activity of the Ca2+-ATPase do not involve aggregation. Biochem. J. 308, 343–346.
Cornea, R. L., and Thomas, D. D. (1994) Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry 33, 2912–2920.
Harroun, T. A., Heller, W. T., Weiss, T. M., Yang, L., and Huang, H. W. (1999) Theoretical analysis of hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76, 3176–3185.
Huang, H. W. (1999) Peptide-lipid interactions and mechanisms of antimicrobial peptides. Novartis Found. Symp. 225, 188–200.
Palczewski, K., Kumasaka, T., Hori, T., Behnke, C. A., Motoshima, H., Fox, B. A., Le Trong, I., Teller, D. C., Okada, T., Stenkamp, R. E., Yamamoto, M., and Miyano, M. (2000) Crystal structure of rhodopsin: A G protein-coupled receptor. Sciece 289 739–745.
Krishna, A. G., Menon, S. T., Terry, T. J., and Sakmar, T. P. (2002) Evidence that helix 8 of rhodopsin acts as a membrane-dependent conformations switch. Biochemistry 41, 8298–8309.
Mozsolits, H., Unabia, S., Ahmad, A., Morton, C. J., Thomas, W. G., and Aguilar, M. I. (2002) Electrostatic and hydrophobic forces tether the proximal region of the Angiotensin II Receptor (AT(1A)) carboxyl terminus to anionic lipids. Biochemistry 41, 7830–7840.
Hurley, J. H., Tsujishita, Y., and Pearson, M. A. (2000) Floundering about at cell membranes: A structural view of phospholipid signaling. Curr. Opin. Struct. Biol. 10, 737–743.
Ordway, R. W., Singer, J. J., and Walsh, J. V., Jr. (1991) Direct regulation of ion channels by fatty acids. Trends Neurosci. 14, 96–100.
Meves, H. (1994) Modulation of ion channels by arachidonic acids. Prog. Neurobiol. 43, 175–186.
Sechi, A. S. and Wehland, J. (2000) The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci. 113 Pt 21, 3685–3695.
McLaughlin, S., Wang, J., Gambhir, A., and Murray, D. (2002) PIP2 and proteins: Interactions, Organization, and Information Flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175.
Hannun, Y. A. and Obeid, L. M. (2002) The Ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850.
Spiegel, S. and Milstien, S. (2002) Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277, 25851–25854.
Hurley, J. H. and Meyer, T. (2001) Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 13, 146–152.
Hilgemann, D. W. (1997) Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu. Rev. Physiol. 59, 193–220.
Jones, J. A. and Hannun, Y. A. (2002) Fight binding inhibition of protein phosphatase-1 by phosphatidic acid. Specificity of inhibition by the phospholipid. J. Biol. Chem. 277, 15530–15538.
Hwang, T. C., Guggino, S. E., and Guggino, W. B. (1990) Direct modulation of secretory chloride channels by arachidonic and other cis unsaturated fatty acids. Proc. Natl. Acad. Sci. USA. 87, 5706–5709.
Schwartz, R. D. and Yu, X. (1992) Inhibition of GABA-gated chloride channel function by arachidonic acid. Brain Res. 585, 405–410.
Ordway, R. W., Walsh, J. V., Jr., and Singer, J. J. (1989) Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science 244, 1176–1179.
Brown, D. A. and London, E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136.
Carter, W. G. and Hakomori, S. (1981) A new cell surface, detergent-insoluble glycoprotein matrix of disulfide bonds in stabilization of the matrix. J. Biol. Chem. 256, 6953–6960.
Okada, Y., Mugnai, G., Bremer, E. G., and Hakomori, S. (1984) Glycosphingolipids in detergent-insoluble substrate attachment matrix (DISAM) prepared from substrate attachment material (SAM). Their possible role in regulating cell adhesion. Exp. Cell Res. 155, 448–456.
Brzustowicz, M. R., Cherezov, V., Caffrey, M., Stillwell, W., and Wassall, S. R. (2002) Molecular organization of cholesterol in polyunsaturated membranes: Microdomain formation. Biophys. J. 82, 285–298.
Radhakrishnan, A. and McConnell, H. M. (2000) Electric field effect on cholesterol-phospholipid complexes. Proc. Natl. Acad. Sci. USA. 97, 1073–1078.
Xu, X. and London, E. (2000) The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39, 843–849.
Fridricksson, E. K., Shipkova, P. A., Sheets, E. D., Holowka, D., Baird, B., and McLafferty, F. W. (1999) Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry 38, 8056–8063.
Schneiter, R., Brugger, B., Sandhoff, R., Zellnig, G., Leber, A., Lampl, M., Athenstaedt, K., Hrastnik, C., Eder, S., Daum, G., Paltauf, F., Wieland, F. T., and Kohlwein, S. D. (1999) Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146, 741–754.
Brown, R. E. (1998) Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. J. Cell Sci. 111, 1–9.
Rietveld, A. and Simons, K. (1998) The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta 1376, 467–479.
Dietrich, C., Bagatolli, L. A., Volovyk, Z. N., Thompson, N. L., Levi, M., Jacobson, K., and Gratton, E. (2001) Lipid rafts reconstituted in model membranes. Biophys. J. 80, 1417–1428.
Moffett, S., Brown, D. A., and Linder, M. E. (2000) Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275, 2191–2198.
Harder, T., Scheiffele, P., Verkade, P., and Simons, K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141, 929–942.
Cheng, P. C., Dykstra, M. L., Mitchell, R. N., and Pierce, S. K. (1999) A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190, 1549–1560.
Janes, P. W., Ley, S. C., and Magee, A. I. (1999) Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447–461.
Anderson, R. G. (1998) The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225.
Dustin, M. L. and Shaw, A. S. (1999) Costimulation: Building an immunological synapse. Science 283, 649–650.
Cheng, P. C., Cherukuri, A., Dykstra, M., Malapati, S., Sproul, T., Chen, M. R., and Pierce, S. K. (2001) Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 13, 107–114.
Cherukuri, A., Dykstra, M., and Pierce, S. K. (2001) Floating the raft hypothesis: Lipid rafts play a role in immune cell activation. Immunity 14, 657–660.
Miceli, M. C., Moran, M., Chung, C. D., Patel, V. P., Low, T., and Zinnanti, W. (2001) Co-stimulation and counter-stimulation: Lipid raft clustering controls TCR signaling and functional outcomes. Semin. Immunol 13, 115–128.
Simons, K. and Ikonen, E. (1997) Functional rafts in cell membranes. Nature 387, 569–572.
Martens, J. R., Navarro-Polanco, R., Coppock, E. A., Nishiyama, A., Parshley, L., Grobaski, T. D., and Tamkun, M. M. (2000) Differential targeting of Shaker-like potassium channels to lipid rafts. J. Biol. Chem. 275, 7443–7446.
Bruses, J. L., Chauvet, N., and Rutishauer, U. (2001) Membrane lipid rafts are necessary for the maintenance of the α7 nicotinic acetlylcholine receptor in somatic spines of ciliary neurons. J. Neurosci. 21, 504–512.
Papazian, D. M., Schwarz, T. L., Tempel, B. L., Jan, Y. N., and Jan, L. Y. (1987) Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science 237, 749–753.
Shieh, C. C., Coghlan, M., Sullivan, J. P., and Gopalakrishnan, M. (2000) Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol. Rev. 52, 557–594.
Choe, S. (2002) Potassium channel structures. Nat. Rev. Neurosci. 3, 115–121.
Yellen, G. (2002) The voltage-gated potassium channels and their relatives. Nature 419, 35–42.
MacKinnon, R., Cohen, S. L., Kuo, A., Lee, A., and Chait, B. T. (1998) Structural conservation in prokaryotic and eukaryotic potassium channels. Science 280, 106–109.
Lu, Z., Klem, A. M., and Ramu, Y. (2001) Ion conduction pore is conserved among potassium channels. Nature 413, 809–813.
Valiyaveetil, F. I., Zhou, Y., and MacKinnon, R. (2002) Lipids in the structure, folding, and function of the KcsA K+ channels. Biochemistry 41, 10771–10777.
Armstrong, C. M. and Hille, B. (1998) Voltage-gated ion channels and electrical excitability. Neuron 20, 371–380.
Yi, B. A., Minor, D. L., Jr., Lin, Y. F., Jan, Y. N., and Jan, L. Y. (2001) Controlling potassium channel activities: Interplay between the membrane and intracellular factors. Proc. Natl. Acad. Sci. USA 98, 11016–11023.
Magoski, N. S., Wilson, G. F., and Kaczmarek, L. K. (2002) Protein kinase modulation of a neuronal cation channel requires protein- protein interactions mediated by an Src homology 3 domain. J. Neurosci. 22, 1–9.
Dascal, N. (2001) Ion-channel regulation by G proteins. Trends Endocrinol. Metab. 12, 391–398.
Mitchell, D. C., Niu, S. L., and Litman, B. J. (2001) Optimization of receptor-G protein coupling by bilayer lipid composition I: Kinetics of rhodopsin-transducin binding. J. Biol. Chem. 276, 42801–42806.
Niu, S. L., Mitchell, D. C., and Litman, B. J. (2001) Optimization of receptor-G protein coupling by bilayer lipid composition II: Formation of metarhodopsin II-transducin complex. J. Biol. Chem. 276, 42807–42811.
Hanlon, M. R., and Wallace, B. A. (2002) Structure and function of voltage-dependent ion channel regulatory beta subunits. Biochemistry 41, 2886–2894.
Martens, J. R., Sakamoto, N., Sullivan, S. A., Grobaski, T. D., and Tamkun, M. M. (2001) Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J. Biol. Chem. 276, 8409–8414.
Gong, J., Xu, J., Bezanilla, M., van Huizen, R., Derin, R., and Li, M. (1999) Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science 285, 1565–1569.
Cook, K. K. and Fadool, D. A. (2002) Two adaptor proteins differentially modulate the phosphorylation and biophysics of Kv1.3 ion channel by SRC kinase. J. Biol. Chem. 277, 13268–13280.
Jiang, Y., Lee, A., Chen, J., Cadene, M., Chait, B. T., and MacKinnon, R. (2002) Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522.
Petrou, S., Ordway, R. W., Hamilton, J. A., Walsh, J. V., Jr., and Singer, J. J. (1994) Structural requirements for charged lipid molecules to directly increase or suppress K+ channel activity in smooth muscle cells. Effects of fatty acids, lysophosphatidate, acyl coenzyme A and sphingosine. J. Gen. Physiol. 103, 471–486.
Denson, D. D., Wang, X., Worrell, R. T., and Eaton, D. C. (2000) Effects of fatty acids on BK channels in GH(3) cells. Am. J. Physiol. Cell Physiol. 279, C1211–1219.
Turnheim, K., Gruber, J., Wachter, C., and Ruiz-Gutierrez, V. (1999) Membrane phospholipid composition affects function of potassium channels from rabbit colon epithelium. Am. J. Physiol. Cell Physiol. 277, C83–90.
Jan, L. Y., and Jan, Y. N. (1997) Cloned potassium channels from eukaryotes and prokaryotes. Annu. Rev. Neurosci. 20, 91–123.
Ruppersberg, J. P. (2000) Intracellular regulation of inward rectifier K+ channels. Pflugers Arch. 441, 1–11.
Fakler, B., Brandle, U., Glowatzki, E., Zenner, H. P., and Ruppersberg, J. P. (1994) Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron 13, 1413–1420.
Huang, C. L., Feng, S., and Hilgemann, D. W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbg. Nature 391, 803–806.
Rohacs, T., Chen, J., Prestwich, G. D., and Logothetis, D. E. (1999) Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J. Biol. Chem. 274, 36065–36072.
Lupu, V. D., Kaznacheyeva, E., Krishna, U. M., Falck, J. R., and Bezprozvanny, I. (1998) Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 273, 14067–14070.
Zhainazarov, A. B. and Ache, B. W. (1999) Effects of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate on a Na+-gated nonselective cation channel. J. Neurosci. 19, 2929–2937.
Ma, H. P., Saxena, S., and Warnock, D. G. (2002) Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J. Biol. Chem. 277, 7641–7644.
Inagaki, N., Gonoi, T., Clement, J. P. t., Namba, N., Inazawa, J., Gonzalez, G., et al. (1995) Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science 270, 1166–1170.
Baukrowitz, T. and Fakler, B. (2000) KATP channels gated by intracellular nucleotides and phospholipids. Eur. J. Biochem. 267, 5842–5848.
Fink, M., Lesage, F., Duprat, F., Heurteaux, C., Reyes, R., Fosset, M., and Lazdunski, M. (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 17, 3297–3308.
Patel, A. J., Honore, E., Maingret, F., Lesage, F., Fink, M., Duprat, F., and Lazdunski, M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290.
Maingret, F., Patel, A. J., Lesage, F., Lazdunski, M., and Honore, E. (2000) Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275, 10128–10133.
Lesage, F., Terrenoire, C., Romey, G., and Lazdunski, M. (2000) Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J. Biol. Chem. 275, 28398–28405.
Arias, H. R. (1997) Topology of ligand binding sites on the nicotinic acetylcholine receptor. Brain Res. Brain Res. Rev. 25, 133–191.
Corringer, P. J., Le Novere, N., and Changeux, J. P. (2000) Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458.
Legendre, P. (2001) The glycinergic inhibitory synapse. Cell Mol. Life Sci. 58, 760–93.
Karlin, A. (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114.
Fong, T. M. and McNamee, M. G. (1986) Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry 25, 830–840.
Criado, M., Eibl, H., and Barrantes, F. J. (1984) Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor- mediated ion translocation. J. Biol. Chem. 259, 9188–9198.
Ochoa, E. L., Dalziel, A. W., and McNamee, M. G. (1983) Reconstitution of acetylcholine receptor function in lipid vesicles of defined composition. Biochim. Biophys. Acta 727, 151–162.
Criado, M., Eibl, H., and Barrantes, F. J. (1982) Effects of lipids on acetylcholine receptor. Essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry 21, 3622–3629.
Jones, O. T., Eubanks, J. H., Earnest J. P., and McNamee, M. G. (1988) A minimum number of lipids are required to support the functional properties of the nicotinic acetylcholine receptor. Biochemistry 27, 3733–3742.
Ellena, J. F., Blazing, M. A., and McNamee, M. G. (1983) Lipid-protein interactions in reconstituted membranes containing acetylcholine receptor. Biochemistry 22, 5523–5535.
Antollini, S. S., Soto, M. A., Bonini de Romanelli, I., Gutierrez-Merino, C., Sotomayor, P., and Barrantes, F. J. (1996) Physical state of bulk and protein-associated lipid in nicotinic acetylcholine receptor-rich membrane studied by laurdan generalized polarization and fluorescence energy transfer. Biophys. J. 70, 1275–1284.
Barrantes, F. The lipid annulus of the nicotinic acetylcholine receptor as a locus of structural-functional interactions. In: A. Watts, ed. Protein-Lipid Interactions, Elsevier Science Publishers B.V., 1993:231–256.
Bhushan, A. and McNamee, M. G. (1990) Differential scanning calorimetry and Fourier transform infrared analysis of lipid-protein interactions involving the nicotinic acetylcholine receptor. Biochim. Biophys. Acta 1027, 93–101.
Narayanaswami, V. and McNamee, M. G. (1993) Protein-lipid interactions and Torpedo californica nicotinic acetylcholine receptor function. 2. Membrane fluidity and ligand-mediated alteration in the accessibility of gamma subunit cysteine residues to cholesterol. Biochemistry 32, 12420–12427.
Dreger, M., Krauss, M., Herrmann, A., and Hucho, F. (1997) Interactions of the nicotinic acetylcholine receptor transmembrane segments with the lipid bilayer in native receptor-rich membranes. Biochemistry 36, 839–847.
Raines, D. E. and Miller, K. W. (1993) The role of charge in lipid selectivity for the nicotinic acetylcholine receptor. Biophys. J. 64, 632–641.
Sunshine, C. and McNamee, M. G. (1992) Lipid modulation of nicotinic acetylcholine receptor function: The role of neutral and negatively charged lipids. Biochim. Biophys. Acta 1108, 240–246.
Bhushan A. and McNamee M. G. (1993) Correlation of phospholipid structure with functional effects on the nicotinic acetylcholine receptor. A modulatory role for phosphatidic acid. Biophys. J. 64, 716–723.
Leibel, W. S., Firestone, L. L., Legler, D. C., Braswell, L. M., and Miller, K. W. (1987) Two pools of cholesterol in acetylcholine receptor-rich membranes from Torpedo. Biochim. Biophys. Acta 897, 249–260.
Jones, O. T. and McNamee, M. G. (1988) Annular and nonannular binding sites for cholesterol associated with the nicotinic acetylcholine receptor. Biochemistry 27, 2364–2374.
Antollini, S. S. and Barrantes, F. J. (1998) Disclosure of discrete sites for phospholipid and sterols at the protein-lipid interface in native acetylcholine receptor-rich membrane. Biochemistry 37, 16653–16662.
Fernandez, A. M., Fernandez-Ballester, G., Ferragut, J. A., and Gonzalez-Ros, J. M. (1993) Labeling of the nicotinic acetylcholine receptor by a photoactivatable steroid probe: Effects of cholesterol and cholinergic ligands. Biochim. Biophys. Acta 1149, 135–144.
Corbin, J., Wang, H. H., and Blanton, M. P. (1998) Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor with [125I]azido-cholesterol. Biochim. Biophys. Acta 1414, 65–74.
Addona, G. H., Sandermann, H., Jr., Kloczewiak, M. A., Husain, S. S., and Miller, K. W. (1998) Where does cholesterol act during activation of the nicotinic acetylcholine receptor? Biochim. Biophys. Acta 1370, 299–309.
Barrantes, F. J., Antollini, S. S., Blanton, M. P., and Prieto, M. (2000) Topography of nicotinic acetylcholine receptor membrane-embededded domains. J. Biol. Chem. 275, 37333–37339.
Popot, J. L., Demel, R. A., Sobel, A., Van Deenen, L. L., and Changeux, J. P. (1978) Interaction of the acetylcholine (nicotinic) receptor protein from Torpedo marmorata electric organ with monolayers of pure lipids. Eur. J. Biochem. 85, 27–42.
Kilian, P. L., Dunlap, C. R., Mueller, P., Schell, M. A., Huganir, R. L., and Racker, E. (1980) Reconstitution of acetylcholine receptor from Torpedo californica with highly purified phospholipids: Effect of alpha-tocopherol, phylloquinone, and other terpenoid quinones. Biochem. Biophys. Res. Commun. 93, 409–414.
Rotstein, N. P., Arias, H. R., Barrantes, F. J., and Aveldano, M. I. (1987) Composition of lipids in elasmobranch electric organ and acetylcholine receptor membranes. J. Neurochem. 49, 1333–1340.
Sunshine, C. and McNamee, M. G. (1994) Lipid modulation of nicotinic acetylcholine receptor function: The role of membrane lipid composition and fluidity. Biochim. Biophys. Acta 1191, 59–64.
Baenziger, J. E., Morris, M. L., Darsaut, T. E., and Ryan, S. E. (2000) Effect of membranelipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 275, 777–784.
Ryan, S. E., Demers, C. N., Chew, J. P., and Baenziger, J. E. (1996) Structural effects of neutral and anionic lipids on the nicotinic acetylcholine receptor. An infrared difference spectroscopy study. J. Biol. Chem. 271, 24590–24597.
Firestone, L. L., Alifimoff, J. K., and Miller, K. W. (1994) Does general anesthetic-induced desensitization of the Torpedo acetylcholine receptor correlate with lipid disordering? Mol. Pharmacol. 46, 508–515.
Fernandez-Ballester, G., Castresana, J., Fernandez, A. M., Arrondo, J. L., Ferragut, J. A., and Gonzalez-Ros, J. M. (1994) A role for cholesterol as a structural effector of the nicotinic acetylcholine receptor. Biochemistry 33, 4065–4071.
Fong, T. M. and McNamee, M. G. (1987) Stabilization of acetylcholine receptor secondary structure by cholesterol and negatively charged phospholipids in membranes. Biochemistry 26, 3871–3880.
Butler, D. H. and McNamee, M. G. (1993) FTIR analysis of nicotinic acetylcholine receptor secondary structure in reconstituted membranes. Biochim. Biophys. Acta 1150, 17–24.
Methot, N., Demers, C. N., and Baenziger, J. E. (1995) Structure of both the ligand- and lipid-dependent channel-inactive states of the nicotinic acetylcholine receptor probed by FTIR spectroscopy and hydrogen exchange. Biochemistry 34, 15142–15149.
McCarthy, M. P., and Stroud, R. M. (1989) Changes in conformation upon agonist binding, and nonequivalent labeling, of the membrane-spanning regions of the nicotinic acetylcholine receptor subunits. J. Biol. Chem. 264, 10911–10916.
McCarthy, M. P. and Moore, M. A. (1992) Effects of lipids and detergents on the conformation of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 267, 7655–7663.
Rankin, S. E., Addona, G. H., Kloczewiak, M. A., Bugge, B., and Miller, K. W. (1997) The cholesterol dependence of activation and fast desensitization of the nicotinic acetylcholine receptor. Biophys. J. 73, 2446–2455.
Raines, D. E. and Krishnan, N. S. (1998) Agonist binding and affinity state transitions in reconstituted nicotinic acetylcholine receptors revealed by single and sequential mixing stopped-flow fluorescence spectroscopies. Biochim. Biophys. Acta 1374, 83–93.
Sooksawate, T. and Simmonds, M. A. (2001) Effects of membrane cholesterol on the sensitivity of the GABAA receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology 40, 178–184.
Sooksawate, T. and Simmonds, M. A. (2001) Influence of membrane cholesterol on modulation of the GABAA receptor by neuroactive steroids and other potentiators. Br. J. Pharmacol. 134, 1303–1311.
Sooksawate, T. and Simmonds, M. A. (1998) Increased membrane cholesterol reduces the potentiation of GABAA currents by neurosteroids in dissociated hippocampal neurones. Neuropharmacology 37, 1103–1110.
Scanlon, S. M., Williams, D. C., and Schloss, P. (2001) Membrane cholesterol modulates serotonin transporter activity. Biochemistry 40, 10507–10513.
Gulbins, E., Szabo, I., Baltzer, K., and Lang, F. (1997) Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc. Natl. Acad. Sci. USA. 94, 7661–7666.
Hilgemann, D. W. and Ball, R. (1996) Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959.
Fan, Z. and Makielski, J. C. (1997) Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272, 5388–5395.
Shyng, S. L. and Nichols, C. G. (1998) Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282, 1138–1141.
Baukrowitz, T., Schulte, U., Oliver, D., Herlitze, S., Krauter, T., Tucker, S. J., Ruppersberg, J. P., and Fakler, B. (1998) PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282, 1141–1144.
Krauter, T., Ruppersberg, J. P., and Baukrowitz, T (2001) Phospholipids as modulators of KATP channels: distinct mechanisms for control of sensitivity to sulphonylureas, K+ channel openers, and ATP. Mol. Pharmacol. 59, 1086–1093.
MacGregor, G. G., Dong, K., Vanoye, C. G., Tang, L., Giebisch, G., and Hebert, S. C. (2002) Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc. Natl. Acad. Sci. USA. 99, 2726–2731.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tillman, T.S., Cascio, M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys 38, 161–190 (2003). https://doi.org/10.1385/CBB:38:2:161
Issue Date:
DOI: https://doi.org/10.1385/CBB:38:2:161