Abstract
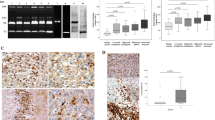
Degradation of the extracellular matrix is a prerequisite for acquisition of the invasive phenotype. Several proteinases released by invading tumor cells appear to participate in the focal degradation of extracellular matrix proteins. Using an enzyme-linked immunosorbent assay, enzymatic assays, Western and Nothern blotting techniques, we determined whether increased levels of the cysteine protease cathepsin B correlated with the progression and invasion of human gliomas. The amount of cathepsin B activity and protein content were highest in glioblastomas, lower in anaplastic astrocytomas and lowest in normal brain tissue and low-grade gliomas. There were significantly higher amounts of Mr 25 000 and 26 000 bands in glioblastoma and anaplastic astrocytoma than in normal brain and low-grade glioma tissue extracts as determined by Western blotting with anti-cathepsin antibodies. In addition, cathepsin B transcripts were overexpressed in anaplastic astrocytoma (about two- to three-fold), in glioblastoma (about eight- to 10-fold), compared with normal brain tissue and low-grade glioma. Immunobistochemical staining for cathepsin B showed intense immunoreactivity in tumor and endothelial cells of glioblastomas and anaplastic astrocytomas but only weak immunoreactivity in low-grade glioma and normal brain tissues. Therefore, we conclude that cathepsin B expression is greatest in highly malignant astrocytomas, especially in glioblastomas, and is correlated with the malignant progression of astrocytomas.
Similar content being viewed by others
References
Russel DE and Rubinstein LJ, 1989, Pathology of the tumors of the nervous system. London: Edward Arnold (ed.) 5th ed.
Gleave JRW, 1986, Surgery for primary brain tumors. In: N. M. Bleehen (ed.), Tumors of the Brain, pp. 101–20. Berlin: Springer-Verlag.
Sheline GE, 1986, Normal tissue tolerance and radiation therapy of gliomas of the adult brain. In: N. M. Bleehen (ed.), Tumors of the Brain, pp. 83–99. Berlin: Springer Verlag.
Workman P, 1986, The pharmacology of brain tumor chemotherapy. In: N. M. Bleehen (ed.). Tumors of the Brain, pp. 183–200. Berlin: Springer-Verlag.
Rutka JT, Myatt CA, Giblin JR, Davis RL and Rosenblum ML, 1987, Distribution of extracellular matrix proteins in primary human brain tumors: an immunohistochemical analysis. Can J Neurol Sci, 14, 25–30.
Dane K, Andreasen PA, Grondahl-Hansen J, et al. 1985, Plasminogen activators, tissue degradation and cancer. Adv Cancer Res, 44, 139–266.
Sloane BF, Honn KV, Sadler, JG et al., 1982, Cathepsin B activity in B16 melanoma cells: a possible marker for metastatic potential. Cancer Res, 42, 980–6.
Eckhout Y and Vaes G, 1977, Further studies on the activation of procollagenase, the latent precursor of bone collagenase: effect of lysosomal cathepsin B, plasmin, and kallikrein and spontaneous activation. Biochem J, 166, 21–31.
Kobayashi H, Schmitt M, Goretzki L, et al. 1991, Cathepsin B efficiently activates the soluble and tumor cell receptor bound form of the proenzyme urokinasetype plasminogen activator (pro uPA). J Biol Chem, 266, 5147–52.
Recklies AD, Tiltman KJ, Stoker AM and Poole RA, 1980, Secretionof proteinases from malignant and non-malignant human breast tissue. Cancer Res, 40, 550–6.
Keppler D, Fondaneche MC, Dalet-Fumeron V, Pagano M and Burtin P, 1988, Immunohistochemical and biochemical study of a cathepsin B-like proteinase in human colonic cancers. Cancer Res, 48, 6855–62.
McCormick D, 1993, Secretion of cathepsin B by human gliomas in vitro. Neuropathol Appl Neurobiol, 19, 146–51.
Bradford MM, 1976, A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem, 72, 248–56.
Barrett AJ and Kirschke H, 1981, Cathepsin B, cathepsin H and cathepsin L. Methods Enzymol, 80, 535–61.
Towbin H, Staehlin T and Grodon J, 1979, Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and applications. Proc Natl Acad Sci USA, 76, 4350–4.
Chomczynski P and Sacchi N, 1987, Single step method of RNA isolation by acid guanidinium thiocyanate phenolchloroform extraction. Anal Biochem, 162, 156–9.
Chan MM-Y and Fong D, 1988, Expression of human cathepsin B protein in Eschericia coli. FEBS Lett, 239, 219–22.
Cao L, Taggart RT, Berquin IM, et al. 1994, Human gastric adenocarcinoma cathepsin B: isolation and sequencing of full-length cDNAs and polymorphisms of the gene. Gene, 139, 163–9.
Gross JR, Behrens DL, Mullins DE, Kornbligh PL and Dexter DL 1988, Plasminogen activator and inhibitor activity in human glioma cells and modulation by sodium butyrate. Cancer Res, 48, 291–6.
Sawaya R, Ramo OJ, Shi ML and Mandybur G, 1991, Biological significance of tissue plasminogen activator content in brain tumors. J Neurosurg, 74, 480–6.
Yamamoto M, Sawaya R, Mohanam S, et al. 1994, Expression and localization of urokinase-type plasminogen activator in human astrocytoma in vivo. Cancer Res, 54, 3656–61.
Rao JS, Steck PA, Kono S, et al. 1994, Role of plasminogen activators and of 92-kDa type IV collagenase in glioblastoma invasion using an in vitro matrigel model. J Neuro-Oncol, 18, 129–38.
Apodaca G, Rutka JT, Bouhana K, et al. 1990, Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res, 50, 2322–9.
Lund-Johansen M, Rucklidge GJ, Milne G and Bjerkvig R, 1991, A metalloproteinase capable of destroying cultured brain tissue isolated from rat glioma cells. Anticancer Res, 11, 1001–6.
Reith A and Ricklidge GJ, 1992, Invasion of brain tissue by primary glioma: evidence for the involvement of urokinase-type plasminogen activator as an activator of the type IV collagenase. Biochem Biophys Res Commun, 186, 348–54.
Caroni P and Schwab ME, 1988, Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol, 106, 1281–8.
Rao JS, Steck PA, Mohanam S, et al. Elevated levels of Mr 92 000 type IV collagenase in human brain tumors. Cancer Res, 53, 2208-11.
McDonald JK and Ellis S, 1975, On the substrate specificity of cathepsin B1 and B2 including a new fluorogenic substrate for cathepsin B. Life Sci, 17, 1269–76.
Olstein AD and Liener IE, 1983, Comparative studies of mouse liver cathepsin B and an analogous tumor thiol proteinase. J Biol Chem, 258, 11049–56.
Home WH, Glossl J and Kresse H, 1987, Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem, 262, 12351–5.
Chan SJ, San Segundo B, McCormick MB and Steiner DF, 1986, Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci USA, 83, 7721–5.
Nishimura Y, Kawabata T and Kato K, 1988, Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Arch Biochem Biophys, 261, 64–71.
Keren Z and Le Grue SJ, 1988, Identification of cell surface cathepsin B-like activity on murine melanomas and fibrosarcomas: modulation of butanol extraction. Cancer Res, 48, 1416–21.
Rozhien J, Robinson D, Stevens MA, et al. 1987, Properties of a plasma membrane-associated cathepsin B-like cystein proteinase in metastatic B16 melanoma variants. Cancer Res, 47, 6620–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sivaparvathi, M., Sawaya, R., Wu Wang, S. et al. Overexpression and localization of cathepsin B during the progression of human gliomas. Clin Exp Metast 13, 49–56 (1995). https://doi.org/10.1007/BF00144018
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00144018