Summary
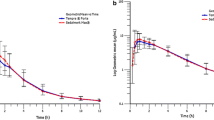
Although the absorption of verapamil is almost complete after oral administration, its bioavailability is low due to extensive hepatic first-pass metabolism. Besides large interindividual differences in first-pass metabolism, pronounced day-to-day intraindividual variations in first-pass metabolism are observed, leading to erroneous results in relative bioavailability studies. Stable isotope techniques, which permit simultaneous administration of a solution and a tablet, can successfully be used to overcome these difficulties. The method has the advantage that two experiments can be carried out in a single test. Furthermore, the number of subjects required in bioavailability studies can be greatly reduced. Using this technique the bioavailability of verapamil tablets (Isoptin® 80) relative to a stable labelled solution of verapamil was found to be 108.1%, with a 95% confidence interval between 89.1 and 127.1%.
Similar content being viewed by others
References
Alvares AP, Kappas A, Eiseman JL, Anderson KE, Pantuck CB, Pantuck EJ, Hsiao K-C, Garland WA, Conncy AH (1979) Intraindividual variation in drug disposition. Clin Pharmacol Ther 26: 407–419
Andreasen F, Boye E, Christoffersen E, Dalsgaard P, Henneberg E, Kallenbach A, Ladefoged S, Lillquist K, Mikkelsen E, Nordero E, Olsen J, Pedersen JK, Pedersen V, Bruun Pedersen G, Schroll J, Schulz H, Seidelin J (1975) Assessment of verapamil in the treatment of angina pectoris. Eur J Cardiol 2: 443–452
Bender F (1970) Die Behandlung tachykarder Arrhythmien und der arteriellen Hypertonie mit Verapamil. Arzneim-Forsch (Drug Res) 20: 1310–1325
Daniel WW (1978) Applied nonparametric statistics, Houghton Mifflin, Boston, pp 135–139
Eichelbaum M, Ende M, Remberg G, Schomerus M, Dengler HJ (1979) The metabolism of DL-(14C)verapamil in man. Drug Metab Dispos 7: 145–148
Eichelbaum M, Somogyi A, Unruh GE von, Dengler HJ (1981) Simultaneous determination of the intravenous and oral pharmacokinetic parameters of D, L-verapamil using stable isotope-labelled verapamil. Eur J Clin Pharmacol 19: 133–137
Fleckenstein A, Tritthart H, Fleckenstein B, Herbst A, Grün G (1969) Eine neue Gruppe kompetitiver Ca+± Antagonisten (Iproveratril, D 600, Prenylamin) mit starken Hemmeffekten auf die elektromechanische Koppelung im Warmblütermyokard. Pflueger's Arch ges Physiol 307: 25–32
Fleckenstein A (1972) Physiologie und Pharmakologie der transmembranären Natrium-, Kalium- und Calcium-Bewegung. Arzneim-Forsch (Drug Res) 22: 2019–2029
Heck Hd'A, Buttrill SE, Flynn NW, Dyer RL. Anbar M, Cairns T, Dighe S, Cabana BE (1979) Bioavailability of imipramine tablets relative to a stable isotope-labelled internal standard: Increasing the power of bioavailability tests. J Pharmacokinet Biopharm 7: 233–248
Kaltenbach M, Hopf R, Keller M (1976) Calciumantagonistische Therapie bei hypertroph-obstruktiver Kardiomyopathie. Dtsch Med Wochenschr 101: 1284–1287
Rosen MR, Wit AL, Hoffmann BF (1975) Electrophysiology and pharmacology of cardiac arrhythmias. IV. Cardiac effects of verapamil. Am Heart J 89: 665–673
Rosing DR, Kent KM, Borer JS, Seides SF, Maron BJ, Epstein SE (1979) Verapamil Therapy: A new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. Circulation 60: 1201–1207
Shomerus M, Spiegelhalder B, Stieren B, Eichelbaum M (1976) Physiological disposition of verapamil in man. Cardiovasc Res 10: 605–612
Singh BN, Ellrodt G, Peter CT (1978) Verapamil: A review of its pharmacological properties and therapeutic use. Drugs 15: 169–197
Spiegelhalder B, Eichelbaum M (1977) Determination of verapamil in human plasma by mass fragmentography using stable-labelled verapamil as internal standard. Arzneim.-Forsch. 27: 94–97
Strong JM, Dutcher JS, Lee W-K, Atkinson AJ (1975) Absolute bioavailability in man of N-acetylprocainamide determined by a novel stable isotope method. Clin Pharmacol Ther 18: 613–622
Wilkinson GR (1975) Pharmacokinetics of drug disposition: Hemodynamic considerations. Annu Rev Pharmacol 15: 11–27
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eichelbaum, M., Dengler, H.J., Somogyi, A. et al. Superiority of stable isotope techniques in the assessment of the bioavailability of drugs undergoing extensive first pass elimination. Eur J Clin Pharmacol 19, 127–131 (1981). https://doi.org/10.1007/BF00568399
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00568399