Summary
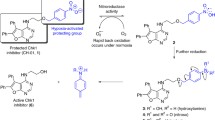
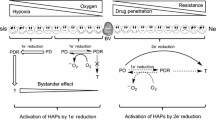
NAD(P)H dependent cytochrome P450's and other haemoproteins under hypoxia, mediate two-electron reduction of a wide range of structurally dissimilar N-oxides to their respective tertiary amines. Metabolic reduction can be utilised, in acute and chronic hypoxia, to convert N-oxides of DNA affinic agents to potent and persistent cytotoxins. In this respect a knowledge of N-oxide bioreduction and the importance of the cationic nature of agents that bind to DNA by intercalation can be combined to rationalise N-oxides as prodrugs of DNA binding agents. The concept is illustrated using the alkylaminoanthraquinones which are a group of cytotoxic agents with DNA binding affinity that is dependent on the cationic nature of these compounds. The actions of the alkylaminoanthraquinones involve drug intercalation into DNA (and double stranded RNA) and inhibition of both DNA and RNA polymerases and topoisomerase Type I and II. A di-N-oxide analogue of mitoxantrone, 1,4-bis{[2-(dimethylamino-N-oxide)ethyl]amino}5,8-dihydroxyanthracene-9,10-dione (AQ4N) has been shown to possess no intrinsic binding affinity for DNA and has low toxicity. Yet in the absence of air AQ4N can be reducedin vitro to a DNA affinic agent with up to 1000-fold increase in cytotoxic potency. Importantly the reduction product, AQ4, is stable under oxic conditions. Studiesin vivo indicate that antitumour activity of AQ4N is manifest under conditions that promote transient hypoxia and/or diminish the oxic tumour fraction. The advantage of utilising the reductive environment of hypoxic tumours to reduce N-oxides is that, unlike conventional bioreductive agents, the resulting products will remain active even if the hypoxia that led to bioactivation is transient or the active compounds, once formed, diffuse away from the hypoxic tumour regions. Furthermore, the DNA affinic nature of the active compounds should ensure their localisation in tumour tissue.
Similar content being viewed by others
References
Overgaard J: Sensitization of hypoxic tumour cells - clinical experience. Int J Radiat Biol 56: 801–811, 1989
Parliament MB, Chapman JD, Urtasun RC, McEwan AJ, Golberg L, Mercer JR, Mannan RH, Wiebe LI: Non-invasive assessment of human tumour hypoxia with123I-iodazomycin arabinoside: preliminary report of a clinical study. Brit J Cancer 65: 90–95, 1992
Freitas I, Baronzio GF: Tumour hypoxia, reoxygenation and oxygenation strategies: possible role in photodynamic therapy. J Photochem Photobiol 11: 3–30, 1991
Rockwell S, Moulder JE: Hypoxic fractions of human tumors xenografted into mice: a review. Int J Radiat Oncol Biol Phys 19: 197–202, 1990
Rockwell S, Moulder JE: Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys 10: 695–712, 1984
Wilson DF, Cerniglia GJ: Localisation of tumors and evolution of their state of oxygenation by phosphorescence imaging. Cancer Res 52: 3988–3993, 1992
Mantyla MJ: Regional blood flow in human tumors. Cancer Res 39: 2304–2306, 1979
Vaupel P, Kallinowski F, Okunieff P: Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumours. A review. Cancer Res 49: 6449–6465, 1989
McLane KE, Fisher J, Ramakrishnan K: Reductive drug metabolism. Drug Metab Revs 14: 741–799, 1983
Riley RJ, Workman P: DT-Diaphorase and cancer chemotherapy. Biochem Pharmacol 43: 1657–1699, 1992
Adams GE, Straford IJ, Wallace RG, Wardman P, Watts ME: The toxicity of nitro compounds towards hypoxic mammalian cellsin vitro: dependence upon reduction potential JNCI 64: 555–560, 1980
Workman P: Keynote address: bioreductive mechanisms. Int J Radiat Oncol Biol Phys 22: 631–637, 1992
King SA, Suffness M, Leyland-Jones B, Hoth DF, O'Dwyer PJ: Indicine N-oxide: clinical use of a pyrollizidine alkaloid. Cancer Treat Reps 71: 517–523, 1987
Zalkow LH, Glinski JA, Gelbaum LT, Fleischmann TJ, McGowan LS, Gordon MM: Synthesis of pyrollizidine alkaloids indicine, intermedine, lycopsamine and analogues and their N-oxides. Potential antitumor agents. J Med Chem 28: 687–694, 1985
Zalkow LH, Glinski JA, Gelbaum LT, Moore D, Melder D, Powis G: Semisynthetic pyrrollizidine alkaloid N-oxide antitumor agents. Esters of Heliotridine. J Med Chem 31: 1520–1526, 1988
Baker MA, Zeman EM, Hirst VK, Brown JM: Metabolism of SR4233 by chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res 48: 5947–5952, 1988
Lloyd RV, Duling DR, Rumyanseva GV, Mason R, Bridson PK: Microsomal reduction of 3-amino-1,2,4-benzotriazine 1,4-dioxide to a free radical. Molec Pharmacol 40: 440–445, 1991
Walton MI, Wolf CR, Workman P: The role of cytochrome P450 and cytochrome P450 reductase in the reductive bioactivation of the novel benzotriazine di-N-oxide hypoxic cytotoxin SR4233 (WIN 59075) by mouse liver. Biochem Pharmacol 44: 251–259, 1992
Riley RJ, Workman P: Enzymology of the reduction of the potent benzotriazine-di-N-oxide hypoxic cell cytotoxin SR 4233 (WIN 59075) by NAD(P)H:(quinone acceptor) oxidoreductase (EC 1.6.99.2) purified from Walker 256 rat tumour cells. Biochem Pharmacol 43: 167–174, 1992
Connors TA: Alkylating prodrugs in cancer chemotherapy. In: Reinhoudt DN, Connors TA, Pinedo HM, van de Poll KW (eds) Structure-activity relationships of antitumour agents. Martin Nijhoff Publ, The Haque/Boston/London 1988, pp 47–57
Cahill A, White INH: Reductive cleavage of N-oxides to cause strand breakage in cell linesin vitro. Biochem Soc Trans 19: 127S, 1991
Panzica RP, Robins RK, Townsend LB: Synthesis and anticancer activity of cytosine arabinoside 3-N-Oxide. J Med Chem 14: 259–260, 1971
Svendsen KR, Overgaard-Hansen K, Frederiksen S, Engelholm SA, Pedersen NT, Windelov LL: Toxicity and metabolism of 3′deoxyribosine N1-oxide in mice and Ehrlich ascites tumor cells. Cancer Chemotherap Pharmacol 30: 86–94, 1992
Jarman M, Leung O-T, Leclercq G, Devleeschouwer N, Stoessel RC, Coombes RC, Skilton RA: Analgues of Tamoxifen: the role of the basic side-chain. Applications of a whole cell oestrogen-receptor binding assay to N-oxides and quarternary salts. Anti-Cancer Drug Design 1: 259–268, 1986
Bickel MH: The pharmacology and biochemistry of N-oxides. Pharmacol Rev 21: 325–355, 1969
Bickel MH: Liver metabolic reactions: Tertiary amine N-dealkylation, tertiary amine N-oxidation, N-oxide reduction and N-oxide N-dealkylation. I. Tricyclic tertiary amine drugs. Archs Biochem Biophys 148: 54–62, 1972
Coccia PF, Westerfield WW: The metabolism of chlorpromazine by liver microsomal enzyme systems. J Pharmacol Exp Ther 157: 446–458, 1967
Patterson LH, Gorrod JW: The metabolism of some 4-substituted N-ethyl N-methylaniline N-oxides. In: Gorrod JW (ed) Biological Oxidation of Nitrogen. Elsevier North Holland, 1978 pp 471-478
Powis G, DeGraw CL, N-oxide reduction by haemoglobin, cytochrome c and ferrous ions. Res Commun Chem Path Pharmacol 30: 143–150, 1980
Kitamura S, Tatsumi K: Involvement of liver aldehyde oxidase in the reduction of nicotinamide N-oxide. Biochem Biophys Res Commun 120: 602–606, 1984
Kitamura S, Wada Y, Tatsumi K: NAD(P)H-dependent reduction of nicotinamide N-oxide by a unique enzyme system consisting of liver microsomal NADPH-cytochrome c reductase and cytosolic aldehyde oxidase. Biochem Biophys Res Commun 125: 1117–1122, 1984
Johnson PRL, Ziegler DM: Properties of a N,N-dimethyl-paminoazobenzene oxide reductase purified from rat liver cytosol. J Biochem Toxicol 1: 15–27, 1986
Sugiura M, Iwasaki K, Kato R: Reduction of tertiary amine N-oxides by liver microsomal P-450. Molec Pharmacol 12: 322–334, 1976
Murry KN, Chaykin S: The enzymatic reduction of nicotinamide N-oxide. J Biol Chem 241: 2029–2034, 1966
Murry KN, Chaykin S: The reduction of nicotinamide N-oxide by xanthine oxidase. J Biol Chem 241: 3468–3473, 1966
Murry KN, Watson GJ, Chaykin S: Catalysis of the direct transfer of oxygen from nicotinamide N-oxide to xanthine by xanthine oxidase. J Biol Chem 241: 4798–4801, 1966
Dajani RM, Gorrod JW, Beckett AH: Reductionin vivo of (-)-nicotine-1′-N-oxide by germ free and conventional rats. Biochem Pharmacol 24: 648–650, 1975
Powis G, Wincentsen L: Pyridine nucleotide cofactor requirements of indicine N-oxide reduction by hepatic microsomal cytochrome P-450. Biochem Pharmacol 29: 347–351, 1980
Sugiura M, Iwasaki K, Kato R: Reduced nicotinamide adenine dinucleotide-dependent reduction of tertiary amine N-oxide by liver microsomal cytochrome P450. Biochem Pharmacol 26: 489–495, 1977
Kato R, Iwasaki K, Noguchi H: Reduction of tertiary amine N-oxides by cytochrome P-450. Mechanism of the stimulatory effect of flavins and methyl viologen. Molec Pharmacol 14: 654–664, 1978
Lindeke B, Paulsen-Sorman U: III Nitrogenous compounds as ligands to haemoporphyrins- The concept of metabolic intermediary complexes. In: Cho AK, Lindeke B (eds) Progress in Basic Clinical Pharmacology Vol 1: Biotransformation of organic nitrogen compounds. Karger Publ, Basel 1988, pp 63–102
Burka L, Guengerich FP, Willard RJ, Macdonald TL: Mechanism of cytochrome P-450 catalysis. Mechanism of N-dealkylation and amine oxide deoxygenation. J Am Chem Soc 107: 2549–2551, 1985
Miyata N, Santa T, Hiroke M: Chemical studies on drug metabolism 6. Deoxygenation of tertiary amine N-oxides and arene oxides by iron (II) porphyrin as a model of cytochrome P-450-dependent reduction. Chem Pharm Bull 32: 377–380, 1984
Powis G, Svingen BA, Degraw C: Iron-EDTA stimulated reduction of indicrine N-oxide by the hepatic microsomal fraction, isolated hepatocytes, and the intact rat. Biochem Pharmacol 31: 293–299, 1982
Wilson WR, van Zijl P, Denny WA: Bis-bioreductive agents as hypoxia selective cytotoxins: nitracrine N-oxide. 7th Int Conf Chem Modifiers of Cancer Treatment E2, 1991
Double JC, Brown JR: The interaction of aminoalkylaminoanthraquinones with deoxyribonucleic acid. J Pharm Pharmacol 27: 502–507, 1975
Double JC, Brown JR: Evaluation of the binding of some substituted anthraquinones and naphthacenequinones to DNA. J Pharm Pharmacol 28: 166–169, 1976
Murdock KC, Child RG, Fabio PF, Angier RB, Wallace RE, Durr FE, Citarella RV: Antitumor agents. 1. 1,4-bis[(aminoalkyl)amino]-9,10-anthracenediones. J Med Chem 22: 1024–1030, 1979
Cheng CC, Zbinden G, Zee-Cheng RK-Y: Comparison of antineoplastic activity of aminoethylaminoanthraquinones and anthracycline antibiotics. J Pharm Sci 68: 393–396, 1979
Johnson RK, Zee-Cheng RK-Y, Lee WW, Acton EM, Henry DW, Cheng CC: Experimental antitumour activity of aminoanthraquinones. Cancer Treat Reps 63: 425–439, 1979
Lown JW, Hanstock CC, Bradley RD, Scraba DG: Interactions of the antitumour agents mitoxantrone and bisantrene with deoxyribonucleic acids studied by electron microscopy. Molec Pharmacol 25: 178–184, 1984
Lown JW, Morgan AR, Yen S-F, Wang YH, Wilson WD: Characteristics of the binding of the anticancer agents mitoxantrone and ametantrone and related structures to deoxyribonucleic acids. Biochem 24: 4028–4035, 1985
Islam SA, Neidle S, Gandecha BM, Partridge M, Patterson LH, Brown JR: Comparative computer graphics and solution studies of the DNA interaction of substituted anthraquinones base on doxorubicin and mitoxantrone. J Med Chem 28: 857–864, 1985
Chen K-X, Gresh N, Pullman B: A theoretical investigation on the sequence-selective binding of mitoxantrone to double stranded DNA. Nucleic Acids Res 14: 3799–3803, 1986
Lown JW, Hanstock CC: High field1NMR analysis of the 1:1 intercalation complex of the antitumour agent mitoxantrone and the DNA duplex [d(CpGpCpGp)]2. J Biol Struct Dyn 2: 1097–1106, 1985
Denny WA, Wakelin LPG: Kinetics of binding of mitoxantrone, ametantrone and analogues to DNA: relationship with binding mode and anti-tumor activity. Anti-Cancer Drug Design 5: 189–200, 1990
Fox KR, Waring MJ, Brown JR, Neidle S: DNA sequence preferences for the anticancer drug mitoxantrone and related anthraquinones revealed by DNAase I footprinting. FEBS Letters 202: 289–293, 1986
Kapuscinski J, Darzynkiewicz Z, Traganos F, Melamed MR: Interactions of a new antitumor agent, 1,4-dihydroxy-5,8-bis[[2-[2-hydroxyethyl)amino]-ethyl]amino]-9-10-anthracenedione, with nucleic acids. Biochem Pharmacol 30: 231–240, 1981
Gandecha BM, Brown JR, Crampton MR: Dissociation kinetics of DNA-anthracycline and DNA-anthraquinone complexes determined by stop flow spectrophotometry. Biochem Pharmacol 34: 733–736, 1985
Krishnamoorthy CR, Yen S-F, Smith JC, Lown JW, Wilson WD: Stopped-flow kinetic analysis of the interaction of anthraquinone anticancer drugs with cald thymus DNA, poly[d(G-C)]poly[d(G-C)] and poly[d(A-T)]poly[d(A-T)]. Biochemistry 25: 5933–5943, 1986
Malhotra D, Hopfinger AJ: Conformational flexibility of dinucleotide dimers unwinding from the B-form to an intercalation structure. Nucleic Acids Res 8: 5289–5300, 1980
Randall K, Broome MG, Hoard WS, Evans SF, Pritchard DF: Experimental therapeutic and biochemical studies of anthracenedione derivatives. In: Rozencweig M (ed) New Anticancer Drugs; Mitoxantrone and Bisantrene. Publ Raven Press, NY 1983, pp 1–28
Traganos F, Evenson DP, Staiano-Coico L, Darzynkiewicz Z, Melamed MR: Action of dihydroxyanthraquinone on cell cycle progression of a variety of cultured mammalian cells. Cancer Res 40: 672–681, 1980
Foye WO, Vajragupta O, Sengupta SK: DNA-binding specificity and RNA polymerase inhibitory activity of bis(aminoalkyl)anthraquinones and bis(methylthio)vinylquinolinium iodides. J Pharm Sci 71: 253–256, 1982
Kapuscinski J, Darzynkiewicz Z: Relationship between the pharmacological activity of ametantrone and mitoxantrone and their ability to condense nucleic acids. Proc Nat Acad Sci USA 83: 6302–6306, 1986
Cohen LF, Glaubiger DL, Kann HE, Kohn KW: Protein associated DNA single strand breaks and cytotoxicity of dihydroxyanthracenedione (DHAD)NSC 301739 in mouse 1210 leukaemia cells. Proc Am Assoc Cancer Res 21: 277, 1980
Bowden GT, Roberts R, Alberts DS, Peng Y-M, Garcia D: Comparative molecular pharmacology in leukemic L1210 cells of the anthracene anticancer drugs mitoxantrone and bisantrene. Cancer Res 45: 4915–4920, 1985
Tewey KM, Rowe TC, Yang L, Haligan BD, Lui LF: Adriamycin induced DNA damage mediated by DNA topoisomerase II. Science 226: 466–468, 1984
Crespi MD, Ivanier SE, Genovese J, Baldi A: Mitoxantrone affects topoisomerase activities in human breast cancer cells. Biochem Biophys Res Commun 136: 521–528, 1986
Lui LF: DNA topoisomerase poisons as antitumour drugs. Annu Rev Biochem 58: 351–375, 1989
Smith PJ: DNA topoisomerase dysfunction: A new goal for antitumour chemotherapy. BioEssays 12: 167–172, 1990
Osheroff N, Zechiedrich EL, Gale KC: Catalytic function of DNA topoisomerase II. BioEssays 13: 269–275, 1991
Roberts RA, Cress AE, Dalton WS: Persistent intracellular binding of mitoxantrone in a human colon carcinoma cell line. Biochem Pharmacol 38: 4283–4290, 1989
Dalton WS, Cress AE, Alberts DS, Trent JM: Cytogenetic and phenotypic analysis of a human colon carcinoma cell line resistant to mitoxantrone. Cancer Res 48: 1882–1888, 1988
Smith PJ, Morgan SA, Fox ME, Watson JV: Mitoxantrone-DNA binding and the induction of topoisomerase II associated DNA damage in multi-drug resistant small cell lung cancer cells. Biochem Pharmacol 40: 2069–2078, 1990
Fox ME, Smith PJ: Long-term trapping of DNA synthesis and the persistence of trapped topoisomerase II complexes in determining the toxicity of the antitumour DNA intercalators mAMSA and mitoxantrone. Cancer Res 50: 5813–5818, 1990
Smith PJ, Sykes HR, Fox ME, Furlong IJ: Subcellular distribution of the anticancer drug mitoxantrone in human and drug-resistant murine cells analyzed by flow cytotometry and confocal microscopy and its relationship to the induction of DNA damage. Cancer Res 52: 4000–4009, 1992
Pincus R, Goldman D: Evidence for impaired mitoxantrone and vinblastine binding in P388 murine leukaemia cells with multidrug resistance. Biochem Pharmacol 40: 2625–2635, 1990
Kharasch ED, Novak RF: Bis(alkylamino)anthracenedione antineoplastic agent metabolic activation by NADPH-cytochrome P450 reductase and NADH dehydrogenase. Arch Biochem Biophys 224: 682–694, 1983
Gianni L, Corden BJ, Myers CE: The biochemical basis of anthracycline toxicity. Rev Biochem Toxicol 5: 1–82, 1983
Fisher GR, Brown JR, Patterson LH: Involvement of hydroxyl radical formation and DNA strand breakage in the cytotoxicity of anthraquinone antitumour agents. Free Rad Res Commun 11: 117–125, 1990
Mimnaugh EG, Dusne L, Atwell J, Myers CE: Differential oxygen radical susceptibility of adriamycin-sensitive and adriamycin-resistant MCF-7 human breast cancer cells: implications for the mechanism of action. Cancer Res 49: 8–15, 1989
Fisher GR, Patterson LH: Lack of involvement of reactive oxygen in the cytotoxicity of mitoxantrone, CI941 and ametantrone in MCF-7 cells: comparison with doxorubicin. Cancer Chemother Pharmacol 30: 451–458, 1992
Fisher GR, Gutierrez PL, Oldcorne MA, Patterson LH: NAD(P)H (quinone acceptor) oxidoreductase (DT-diaphorase) mediated two electron reduction of anthraquinonebased antitumour agents and generation of hydroxyl radicals. Biochem Pharmacol 43: 575–585, 1992
Reska K, Kolodziejczyk P, Lown JW: Horseradish peroxidase-catalysed oxidation of mitoxantrone: spectrophotometric and electron paramagnetic studies. Free Radicals Biol Med 2: 25, 1986
Reska K, Hartley JA, Kolodziejczyk P, Lown JW: Interaction of the peroxidase-derived metabolite of mitoxantrone with nucleic acids: evidence for covalent binding of14C-labelled drug. Biochem Pharmacol 38: 4253–4260, 1989
Fisher GR, Patterson LH: DNA strand breakage by peroxidase-activated mitoxantrone J. Pharm Pharmacol 43: 65–68, 1990
Cheng CC, Zee-Cheng RK-Y: The design, synthesis and development of a new class of potent antineoplastic anthraquinones. In: Ellis GP, West GB (eds) Progress in Medicinal Chemistry Vol 20. Elsevier Science Publ, 1983, pp 83–115
Martelli S, Dzieduszcka M, Stefanska B, Bontemps-Gracz M, Borowski E: Synthesis and antineoplastic activity of 1,4-Bis(aminoalkanamido)9,10-anthracenediones. J Med Chem 31: 1956–1959, 1988
Zee-Cheng RK-Y, Cheng CC: Structure-activity relationship study of anthraquinones: 1,4-dihydroxy-5,8-bis[[2-(2-hydroxyethoxy)ethyl]amino]-9,10-anthracenedione, an analog of an established antineoplastic agent. J Pharm Sci 71: 708–709, 1982
Patterson LH, Maine JE, Cairns DC, Craven MR, Bennett N, Fisher GR, Ruparelia K, Giles Y: N-oxides of DNA affinic agents as bioreductively activated prodrugs. Proc Am Soc Cancer Res 33: 2571, 1992
Patterson LH: Anthraquinone anticancer compounds with (disubstituted amino-N-oxide)alkylamino substituent. UK Patent GB 2: 237–283, 1989
Patterson LH: Anticancer compounds (anthrapyrazole N-oxides). UK Patent GB 22546148A, 1992
Patterson LH: Anticancer compounds (anthracene N-oxides). UK Patent GB 22546143A, 1992
Author information
Authors and Affiliations
Additional information
Nomenclature and properties of N-oxides
The term N-oxide is a functionality used to describe the oxide of a tertiary amine created by addition of atomic oxygen to the lone pair electrons of the nitrogen atom. Aromatic, arylaliphatic and aliphatic tertiary amines can all form N-oxides. The resulting dative covalent nitrogen-oxygen bond is semi-polar and can be described formally as N+-O−. N-Oxides are weaker bases than the respective parent tertiary amines generally by about 2 pKa units. However such N-oxides are still sufficiently basic to protonate at acid pH and form addition salts e.g. hydrochloride or acetate.
Rights and permissions
About this article
Cite this article
Patterson, L.H. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metast Rev 12, 119–134 (1993). https://doi.org/10.1007/BF00689805
Issue Date:
DOI: https://doi.org/10.1007/BF00689805