Summary
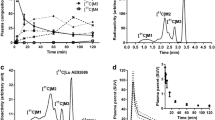
The regional brain kinetics of (β-11C)-L-dopa and 6-fluoro-(β-11C)-L-dopa was measured in six Rhesus monkeys using positron emission tomography (PET). Radioactivity accumulated specifically in the striatal region and the increase in L-dopa-derived radioactivity utilization with time was calculated using surrounding brain as a reference area, this being devoid of dopaminergic activity. The rate constant for selective striatal utilization i.e. grossly decarboxylation was 0.0110 ± 0.0007 (S.D) and 0.0057 ± 0.0006 min1 for (β-11C)-L-dopa and 6-fluoro-(β-11C)-L-dopa, respectively. After pre-treatment of the monkeys with the peripherally and centrally active catecholamine-O-methyl transferase (COMT) inhibitor Ro 40-7592 10 mg/kg, the decarboxylation rate remained unchanged (0.0112 ± 0.0015 min-1) for (β11C)-L-dopa, whereas an increase in rate was measured for 6-fluoro-(β-11C)L-dopa (0.0092 ± 0.0015 min−1). Differences in the distribution of radiolabelled metabolites i.e. the corresponding O-methyl-L-dopa in the reference area is most probably the reason for the difference in calculated decarboxylation rate seen between the radiotracers. The higher decarboxylation rate measured for 6-fluoro-(β-11C)-L-dopa after blockade of COMT shows that the radiolabelled metabolites i.e. 6-fluoro-O-methyl-(β-11C)-L-dopa significantly contributes to background radioactivity.
Similar content being viewed by others
References
Bjurling P, Watanabe Y, Oka S, Nagasawa T, Yamada H, Langström B (1990) Multienzymatic synthesis of β-11C-labelled L-tyrosine and L-dopa. Acta Chem Scand 44: 183–188
Bjurling P, Malmborg P, Långström B (1991) Enzymatic synthesis of some 11C-analogues of L-tyrosine and L-tryptophan. J Label Comp Radiopharm XXX: 399–400
Boyes BE, Cumming P, Martin WRW, McGeer EG (1986) Determination of plasma (18F)-6-fluoro-dopa during positron emission tomography: elimination and metabolism in carbidopa treated subjects. Life Sci 39: 2243–2252
Calne DB, Langston JW, Martin WRW, Stoessl J, Ruth TJ, Adam MJ, Pate BD, Schulzer M (1985) Positron emission tomography after MPTP: observation relating to the cause of Parkinson's disease. Nature 317: 246–248
Cumming P, Boyes BE, Martin WRW, Adam M (1987) The metabolism of (18F)-fluoro-L-3,4-dihydroxiphenylalanine in the hooded rat. J Neurochem 48: 601–608
Ferrini R, Glaser A (1964) In vitro decarboxylation of new phenylalanine derivatives. Biochem Pharmacol 13: 798–800.
Firnau G, Sood S, Chirakal R, Nahmias C, Garnett ES (1987) Cerebral metabolism of 6-(18F)-fluoro-L-3,4-dihydrophenylalanine in the primate. J Neurochem 48: 1077–1082
Firnau G, Sood S, Chirakal R, Nahmias C, Garnett ES (1988) Metabolites of (18F)-fluoro-L-dopa in human blood. J Nucl Med 29: 363–369
Garnett ES, Firnau G, Nahmias C, Chirakal R (1983a) Striatal dopamine metabolism in living monkeys examined by positron emission tomography. Brain Res 280: 169–171
Garnett ES, Firnau G, Nahmias C (1983b) Dopamine visualized in the basal ganglia of living man. Nature 305: 137–138
Garnett ES, Nahmias C, Firnau G (1983c) Central dopaminergic pathways in hemiparkinsonism examined by positron emission tomography. Can J Neurol Sci 11: 174–179
Gjedde A (1982) Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res Rev 4: 237–274
Hartvig P, Ågren H, Reibring L, Tedroff J, Bjurling P, Kihlberg T, Långström B (1991) Brain kinetics of L-(β-11C)-dopa in humans studied by positron emission tomography. J Neural Transm 86: 25–41
Home MK, Cheng CH, Wooten GF (1984) The cerebral metabolism of L-dihydroxiphenylalanine. Pharmacology 28: 12–26
Leenders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, Nahmias C, Jones T, Marsden CD (1986) Brain dopamine metabolism in patients with Parkinson's disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry 49: 853–860
Liskowski D, Potter LT (1985) The pre-positron emission tomography study of L-3,4-dihydroxy-(3H)-phenylalanine distribution in the rat. Neurosci Lett 53: 161–167
Melega WP, Luxen A, Perlmutter MM, Nissenson CHK, Phelps ME, Barrio JR (1990) Comparative in vivo metabolism of 6-(18F)-fluoro-L-dopa and (3H)-L-dopa in rats. Biochem Pharmacol 39: 1853–1860
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3: 1–7
Tedroff J, Aquilonius SM, Hartvig P, Bjurling P, Långström B (1991 a) Estimation of regional cerebral utilization of (11C)-L-3,4-dihydroxy-phenylalanine (dopa) in the primate by positron emission tomography. Acta Neurol Scand (in press)
Tedroff J, Aquilonius SM, Hartvig P, Bredberg Eva, Bjurling P, Långström B (1991b) Cerebral utilization of β-(11C)-L-dopa in Parkinsons disease measured by positron emission tomography-relations to motor response. Acta Neurol Scand (in press)
Tedroff J, Hartvig P, Bjurling P, Andersson Y, Antoni G, Långström B (1991c) Central action of benzerazide after COMT inhibition demonstrated in vivo by PET. J Neural Transm [Gen Sect] 85: 11–17
Zürcher G, Keller HH, Kettler R, Borgulya J, Bonetti EP, Eigenman R, Da Prada M (1990) Ro 40-7592, a novel, very potent and orally active inhibitor of catechol-Omethyl transferase: a pharmacological study in rats. In: Korczyn AD, Melamed E, Youdim MBH (eds) Parkinson's disease: anatomy pathology and therapy. Raven Press, New York, pp 497–503 (Advances in Neurology, vol 53)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hartvig, P., Lindner, K.J., Tedroff, J. et al. Regional brain kinetics of 6-fluoro-(β-11C)-L-dopa and (β-11C)-L-dopa following COMT inhibition. A study in vivo using positron emission tomography. J. Neural Transmission 87, 15–22 (1992). https://doi.org/10.1007/BF01253107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01253107