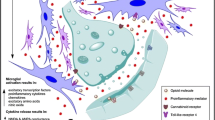
Abstract
Spinal acute opioid tolerance remains mechanistically undercharacterized. Expanded clinical use of direct spinal administration of opioids and other analgesics indicates that studies to further understand spinal mechanisms of analgesic tolerance are warranted. Rodent models of spinal administration facilitate this objective. Specifically, acute spinal opioid tolerance in mice presents a plasticity-dependent, rapid, and efficient opportunity for evaluation of novel clinical agents. Similarities between the pharmacology of acute and chronic spinal opioid tolerance, neuropathic pain, and learning and memory suggest that this model may serve as a high through-put predictor of bioactivity of novel plasticity-modifying compounds.
Similar content being viewed by others
References
Abraham WC, Mason SE. Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res 461/1:40–46;1988.
Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol 69:285–287;1995.
Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW. Nitric oxide and opioid tolerance. Neuropharmacology 33:1463–1470;1994.
Bannerman DM, Chapman PF, Kelly PA, Butcher SP, Morris RG. Inhibition of nitric oxide synthase does not prevent the induction of long-term potentiation in vivo. J Neurosci 14:7415–7425;1994.
Behar M, Magora F, Olshwang D, Davidson JT. Epidural morphine in treatment of pain. Lancet i:527–529;1979.
Ben-Eliyahu S, Marek P, Vaccarino AL, Mogil JS, Sternberg WF, Liebeskind JC. The NMDA receptor antagonist MK-801 prevents long-lasting non-associative morphine tolerance in the rat. Brain Res 575:304–308;1992.
Bennett GJ, Xie Y-K. A peripheral neuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107;1988.
Bethea JR, Gillespie GY, Benveniste EN. Interleukin-1 beta induction of TNF-alpha gene expression: Involvement of protein kinase C. J Cell Physiol 152/2:264–273;1992.
Bhargava HN. Attenuation of tolerance to, and physical dependence on, morphine in the rat by inhibition of nitric oxide synthase. Gen Pharmacol 26:1049–1053;1995.
Bhargava HN, Zhao GM. Effects of N-methyl-D-aspartate receptor antagonists on the analgesia and tolerance toD-Ala(2), Glu(4) deltorphin II, a delta(2)-opioid receptor agonist in mice. Brain Res 719/1–2:56–61;1996.
Bilsky EJ, Inturrisi CE, Sadee W, Hruby VJ, Porreca F. Competitive and noncompetitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain 68/2–3:229–237;1996.
Bliss TVP, Collingridge GL. A synaptic model of memory — Long-term potentiation in the hippocampus. Nature 361:31–39;1993.
Bock J, Wolf A, Braun K. Influence of the N-methyl-D-aspartate receptor antagonistDL-2-amino-5-phosphonovaleric acid on auditory filial imprinting in the domestic chick. Neurobiol Learn Mem 65/2:177–188;1996.
Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA 90:9191–9194;1993.
Bohme GA, Bon C, Stutzmann JM, Doble A, Blanchard JC. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol 199:379–381;1991.
Bordi F, Marcon C, Chiamulera C, Reggiani A. Effects of the metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology 35:1557–1565;1996.
Borgerding RA, Murphy S. Expression of inducible nitric oxide synthase in cerebral endothelial cells is regulated by cytokine-activated astrocytes. J Neurochem 65/3:1342–1347;1995.
Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav Neurosci 110/1:86–102;1996.
Caramanos Z, Shapiro ML. Spatial memory and N-methyl-D-aspartate receptor antagonists APV and MK-801: Memory impairments depend on familiarity with the environment, drug dose, and training duration. Behav Neurosci 108/1:30–43;1994.
Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther 280:829–838;1997.
Coombs DW, Saunders RL, Lachance D, Savage S, Ragnarsson TS, Jensen LE. Intrathecal morphine tolerance: Use of intrathecal clonidine, DADLE, and intraventricular morphine. Anesthesiology 62:358–363;1985.
Cousins MJ, Mather LE, Glynn CJ, Wilson PR, Graham JR. Selective spinal analgesia. Lancet i:1141–1142;1979.
Cox BM, Ginsburg M, Willis J. The offset of morphine tolerance in rats and mice. Br J Pharmacol 53:383–391;1975.
Danysz W, Wroblewski JT, Costa E. Learning impairment in rats by N-methyl-D-aspartate receptor antagonists. Neuropharmacology 27:653–656;1988.
DeLander GE, Portoghese PS, Takemori AE. Role of spinal mu opioid receptors in the development of morphine tolerance and dependence. J Pharmacol Exp Ther 231/1:91–96;1984.
Delander GE, Takemori AE. Spinal antagonism of tolerance and dependence induced by systemically administered morphine. Eur J Pharmacol 94/1–2:35–42;1983.
Doyle C, Holscher C, Rowan MJ, Anwyl R. The selective neuronal NO synthase inhibitor 7-nitro-indazole blocks both long-term potentiation and depotentiation of field EPSPs in rat hippocampal CA1 in vivo. J Neurosci 16:418–424;1996.
Elliot K, Hynansky A, Inturrisi CE. Dextromethorphan attenuates and reverses analgesic tolerance to morphine. Pain 59:361–368;1994.
Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the muopioid morphine but not kappa opioids. Pain 56:69–75;1994.
Fairbanks CA, Stone LS, Kitto KF, Wilcox GL. The anti-proinflammatory cytokines IL-10 and IL-1ra increase the potency of μ opioids morphine and DAMGO in morphine tolerant and non-tolerant mice. Soc Neurosci Abstr 23:1121;1997.
Fairbanks CA, Wilcox GL. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J Pharmacol Exp Ther 282:1408–1417;1997.
Faraci FM, Brian JE Jr. 7-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-D-aspartate. Stroke 26:2172–2175;1995.
Feinberg MP, Cochin J. Studies on tolerance. II. The effect of timing on inhibition of tolerance to morphine by cycloheximide. J Pharmacol Exp Ther 203:332–339;1977.
Fin C, da Cunha C, Bromberg E, Schmitz PK, Bianchin M, Medina JH, Izquierdo I. Experiments suggesting a role for nitric oxide in the hippocampus in memory processes. Neurobiol Learn Mem 63/2:113–115;1995.
Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 316:247–249;1996.
Hargreaves EL, Cote D, Shapiro ML. A dose of MK801 previously shown to impair spatial learning in the radial maze attenuates primed burst potentiation in the dentate gyrus of freely moving rats. Behav Neurosci 111/1:35–48;1997.
Huang CC, Hsu KS. Nitric oxide signalling is required for the generation of anoxia-induced long-term potentiation in the hippocampus. Eur J Neurosci 9:2202–2206;1997.
Huidobro F, Huidobro-Toro JP, Leong Way E. Studies on tolerance development to single doses of morphine in mice. J Pharmacol Exp Ther 198:318–329;1976.
Hylden JLK, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol 67:313–316;1980.
Hylden JLK, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res 217:212–215;1981.
Iga Y, Yoshioka M, Togashi H, Saito H. Inhibitory action of N-omega-nitro-L-arginine methyl ester on in vivo long-term potentiation in the rat dentate gyrus. Eur J Pharmacol 238:395–398;1993.
Inoue T, Mashimo T, Shibata M, Shibuta S, Yoshiya I. Rapid development of nitric oxide-induced hyperalgesia depends on an alternate to the cGMP-mediated pathway in the rat neuropathic pain model. Brain Res 792/2:263–270;1998.
Izquierdo I. Pharmacological evidence for a role of long-term potentiation in memory. FASEB J 8:1139–1145;1994.
Jaffe K, Blanco ME. Involvement of amino acids, opioids, nitric oxide, and NMDA receptors in learning and memory consolidation in crickets. Pharmacol Biochem Behav 47:493–496;1994.
Janssen PA, Niemegeers CJE, Dony JGH. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneimittelforschung 13:502–507;1963.
Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 273:1405–1409;1996.
Kest B, McLemore G, Kao B, Inturrisi CE. The competitive alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. J Pharmacol Exp Ther 283:1249–1255;1997.
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363;1992.
Kolesnikov Y, Jain S, Pasternak GW. Modulation of opioid analgesia by agmatine. Eur J Pharmacol 296:17–22;1996.
Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not kappa opioids by a nitric oxide synthesis inhibitor. Proc Natl Acad Sci USA 90:5162–5166;1993.
Kolesnikov YA, Pick CG, Pasternak GW. N-G-Nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol 221:399–400;1992.
Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: Involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience 84:583–589;1998.
Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain 84:159–167;2000.
Laughlin TM, Stone LS, Schreiber KL, Wilcox GL. Role of inducible nitric oxide synthase in dynorphin-induced allodynia: A central model of neuropathic pain. Soc Neurosci Abstr 24:638;1998.
Laughlin TM, Vanderah T, Lashbrook JM, Nichols ML, Ossipov MH, Porecca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: Involvement of NMDA but not opioid receptors. Pain 72:253–260;1997.
Le AD, Kalant H. Influence of intoxicated practice on the development of acute tolerance to the motor impairment effect of ethanol. Psychopharmacology 106:572–576;1992.
Le AD, Mana M, Quan B, Kalant H. Differential development of acute tolerance to the motor impairment and anticonvulsant effects of ethanol. Psychopharmacology 109/1–2:107–111;1992.
Leem JW, Choi EJ, Park ES, Paik KS. N-Methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett 211/1:37–40;1996.
Leung LS, Shen B. Long-term potentiation in hippocampal CA1: Effects of afterdischarges, NMDA antagonists, and anticonvulsants. Exp Neurol 119/2:205–214;1993.
Li HB, Matsumoto K, Tohda M, Yamamoto M, Watanabe H. NMDA — but not AMPA — receptor antagonists augment scopolamine-induced spatial cognitive deficit of rats in a radial maze task. Brain Res 725/2:268–271;1996.
Majeed NH, Przewlocka B, Machelska H, Przewlocki R. Inhibition of nitric oxide synthase attenuates the development of morphine tolerance and dependence in mice. Neuropharmacology 33/2:189–192;1994.
Malenfant SA, O'Hearn S, Fleming AS. MK801, an NMDA antagonist, blocks acquisition of a spatial task but does not block maternal experience effects. Physiol Behav 49/6:1129–1137;1991.
Marek P, Ben-Eliyahu S, Gold M, Liebeskind JC. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res 547:77–81;1991.
Markevich V, Scorsa AM, Dawe GS, Stephenson JD. Cholinergic facilitation and inhibition of long-term potentiation of CA1 in the urethane-anaesthetized rats. Brain Res 754/1–2:95–102;1997.
Mesches MH, Bianchin M, McGaugh JL. The effects of intra-amygdala infusion of the AMPA receptor antagonist CNQX on retention performance following aversive training. Neurobiol Learn Mem 66:324–340;1996.
Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32:197–200;1994.
Mizutani A, Saito H, Abe K. Involvement of nitric oxide in long-term potentiation in the dentate gyrus in vivo. Brain Res 605:309–311;1993.
Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception. I. Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80/1–2:67–82;1999.
Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774–776;1986.
Müller D, Bittar P, Boddeke H. Induction of stable long-term potentiation in the presence of the protein kinase C antagonist staurosporine. Neurosci Lett 135:18–22;1992.
Ng KT, O'Dowd BS, Rickard NS, Robinson SR, Gibbs ME, Rainey C, Zhao WQ, Sedman GL, Hertz L. Complex roles of glutamate in the Gibbs-Ng model of one-trial aversive learning in the new-born chick. Neurosci Biobehav Rev 21/1:45–54;1997.
Nicolarakis PJ, Lin YQ, Bennett MR. Effect of nitric oxide synthase inhibition on long-term potentiation at associational-commissural and mossy fibre synapses on CA3 pyramidal neurones. Br J Pharmacol 111:521–524;1994.
Onofrio BM, Yaksh TL, Arnold PG. Continuous low-dose intrathecal morphine administration in the treatment of chronic pain of malignant origin. Mayo Clin Proc 56:516–520;1981.
Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci 13:5228–5241;1993.
Riancho JA, Zarrabeitia MT, Fernandez-Luna JL, Gonzalez-Macias J. Mechanisms controlling nitric oxide synthesis in osteoblasts. Mol Cell Endocrinol 107/1:87–92;1995.
Rogan MT, Staubli UV, LeDoux JE. AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci 17:5928–5935;1997.
Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci 17:5129–5135;1997.
Samuelsson H, Malmberg F, Eriksson M, Hedner T. Outcomes of epidural morphine treatment in cancer pain: Nine years of clinical experience. J Pain Symptom Manage 10/2:105–112;1995.
Schmidt CF, Livingston AE. The action of morphine on the mammalian circulation. J Pharmacol Exp Ther 47:411–442;1933.
Schmidt CF, Livingston AE. The relation of dosage to the development of tolerance to morphine in dogs. J Pharmacol Exp Ther 47:443–470;1933.
Schneemann M, Schoedon G, Frei K, Schaffner A. Immunovascular communication: Activation and deactivation of murine endothelial cell nitric oxide synthase by cytokines. Immunol Lett 35/2:159–162;1993.
Shimoyama N, Shimoyama M, Inturrisi CE, Elliott KJ. Ketamine attenuates and reverses morphine tolerance in rodents. Anesthesiology 85/6:1357–1366;1966.
Simmons ML, Murphy S. Cytokines regulateL-arginine-dependent cyclic GMP production in rat glial cells. Eur J Neurosci 5:825–831;1993.
Siuciak JA, Altar CA, Wiegand SJ, Lindsay RM. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res 633/1–2:326–330;1994.
Siuciak JA, Wong V, Pearsall D, Wiegand SJ, Lindsay RM. BDNF produces analgesia in the formalin test and modifies neuropeptide levels in rat brain and spinal cord areas associated with nociception. Eur J Neurosci 7:663–670;1995.
Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MM, Wilcox GL. Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and -2. Neuroreport 8:3131–3135;1997.
Svendsen F, Tjolsen A, Hole K. AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. Neuroreport 9:1185–1190;1998.
Tallarida RJ, Murray RB. Manual of Pharmacological Calculations with Computer Programs. New York, Springer, 26–31;1987.
Tiseo PJ, Cheng J, Pasternak GW, Inturrisi CE. Modulation of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY274614: Assessment of opioid receptor changes. J Pharmacol Exp Ther 268/1:195–201;1994.
Tiseo PJ, Inturrisi CE. Attenuation and reversal of morphine tolerance by the competitive NMDA receptor antagonist, LY274614. J Pharmacol Exp Ther 264:1090–1096;1993.
Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87;1991.
Vanderah T, Laughlin T, Lashbrook J, Nichols M, Wilcox G, Ossipov M, Malan T, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: Blockade by MK-801 but not naloxone. Pain 68:275–281;1996.
Vaupel DB, Kimes AS, London ED. Comparison of 7-nitro indazole with other nitric oxide synthase inhibitors as attenuators of opioid withdrawal. Psychopharmacology 118:361–368;1995.
Venero C, Sandi C. Effects of NMDA and AMPA receptor antagonists on corticosterone facilitation of long-term memory in the chick. Eur J Neurosci 9:1923–1928;1997.
Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology 50/2:149–151;1979.
Wiesenfeld-Hallin Z, Xu XJ, Hao JX, Hokfelt T. The behavioural effects of intrathecal galanin on tests of thermal and mechanical nociception in the rat. Acta Physiol Scand 147:457–458;1993.
Williams JH, Ko YG, Nayak A, Errington ML, Murphy KP, Bliss TV. The suppression of long-term potentiation in rat hippocampus by inhibitors of nitric oxide synthase is temperature and age dependent. Neuron 11:877–884;1993.
Wong CS, Cherng CH, Tung CS. Intrathecal administration of excitatory amino acid receptor antagonists or nitric oxide synthase inhibitor reduced autotomy behavior in rats. Anesth Analg 87:605–608;1998.
Yaksh TL, Huang SP, Rudy TA. The direct and specific opiate-like effect of met(5)-enkephalin and analogues on the spinal cord. Neuroscience 2:593–596;1977.
Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036;1976.
Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 202:411–428;1977.
Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain 49:121–128;1992.
Yang XC, Reis DL. Agmatin selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther 288:544–549;1999.
Yang XC, Reis DL. Agmatine selectively blocks the NMDA subclass of glutamate receptor channels in cultured mouse hippocampal neurons. Neurosci Abstr 23:1763;1997.
Zajaczkowski W, Frankiewicz T, Parsons CG, Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology 36:961–971;1997.
Zhao GM, Bhargava HN. Nitric oxide synthase inhibition attenuates tolerance to morphine but not to [D-Ala(2),Glu(4)] deltorphin II, a delta(2)-opioid receptor agonist in mice. Peptides 17:619–623;1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fairbanks, C.A., Wilcox, G.L. Spinal plasticity of acute opioid tolerance. J Biomed Sci 7, 200–212 (2000). https://doi.org/10.1007/BF02255467
Issue Date:
DOI: https://doi.org/10.1007/BF02255467